Figure 9.
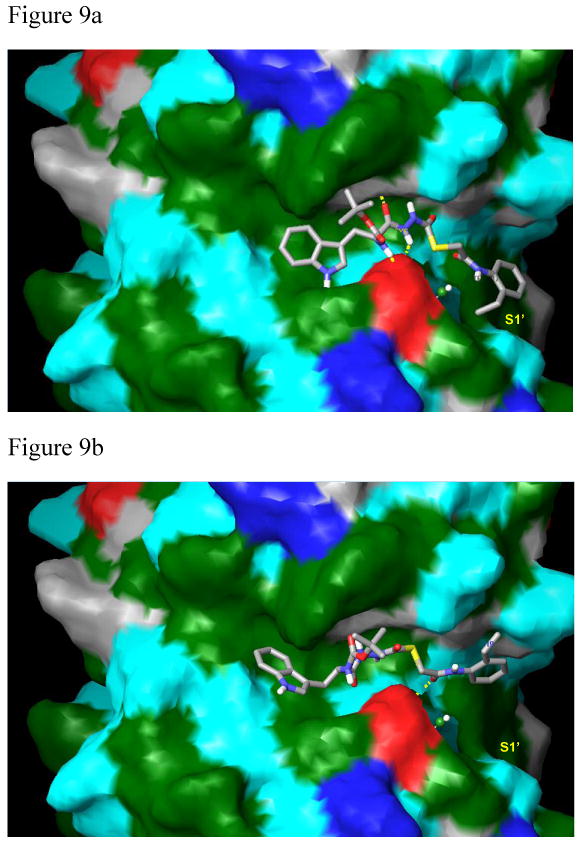
Compounds 1 (9a) and 2 (9b) bound to papain. The protein surface was calculated in Maestro (Schrodinger, Inc.) with molecular properties color-coded as follows: green: strongly hydrophobic; red: negatively charged (Asp158 residue); dark blue: positively charged; cyan and grey: hydrophilic. 9a. Compound 1 binds via a strong hydrophobic interaction within the S1′ subsite consisting of an aromatic/aromatic interaction between Trp177 and the 2-ethylphenyl anilide group of the ligand. In addition, there are strong hydrophobic interactions between the indole of the ligand within the S2 subsite and between the tert-butoxy group in the S3 subsite (XP Glide score: -9.04 kcal/mol). 9b. The phenethyl group of 2 lies completely outside of the S1′ hydrophobic binding pocket and the indole of 2 looses significant hydrophobic contacts in the S2 subsite. The tert-butoxy group of the ligand also looses hydrophobic contacts within the S3 subsite. Critical hydrogen bonds are absent in this complex, resulting in the lower XP Glide score of -7.03 kcal/mol, and the weakened biological activity (33 μM).
Figures 9a and 9b. Compounds 1 and 2 shown in Connolly surface models of the protein.
