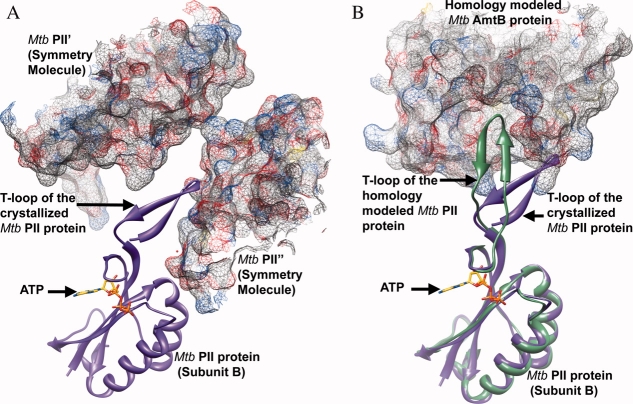
Figure 4.
A) Part of the T-loop of subunit B of the Mtb PII protein is stabilized by its interaction with the symmetry related molecules Mtb PII′ and Mtb PII”. Subunit B of Mtb PII is shown in purple ribbon representation along with the bound ATP in stick representation. The symmetry related molecules Mtb PII′ and Mtb PII” are shown in mesh representation with 50% transparency.(B) Homology model of Mtb AmtB and Mtb PII:ATP based on the crystal structure of the E. coli GlnK:AmtB complex.16,34 AmtB is shown in mesh representation with 50% transparency and PII is shown in green ribbon representation. Subunit B of the crystallized Mtb PII:ATP structure is superposed on to the homology modeled Mtb PII structure and shown in purple ribbon representation along with the bound ATP in stick representation. The superposition highlights the ∼45° movement required by the T-loop of the crystallized Mtb PII protein to interact with the T-loop binding site of the Mtb AmtB protein.