Figure 3.
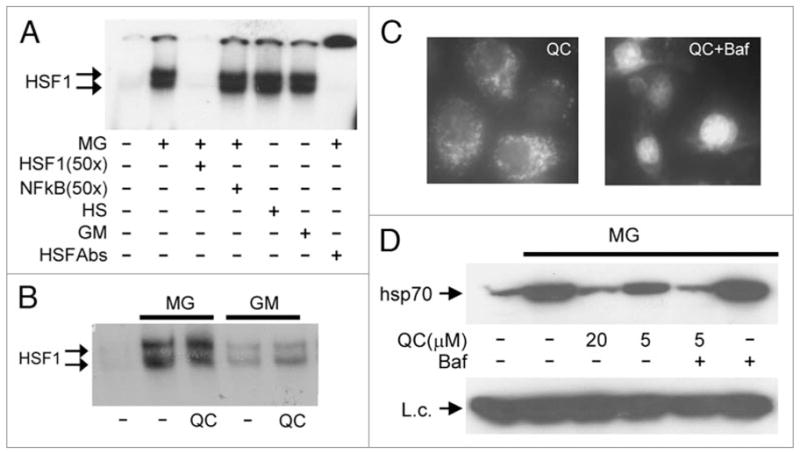
Aminoacridines act downstream of HSF1 activation and translocation to the nucleus. (A) Treatment of HeLa cells with MG132, 17-DMAG, or heat shock induces HSF1 DNA binding activity. Electrophoretic mobility shift assays (EMSA) were performed with cytoplasmic extracts from HeLa cells left untreated, treated with 5 μM MG132 (MG) or 1 μM 17-DMAG (GM) for 4 h, or treated with heat shock (43°C) for 1 h. Complex formation between HSF1 and a p32-labeled oligonucleotide probe containing an HSF1 binding site was inhibited by a 50x excess of the same unlabeled oligonucleotide (lane 3), but not by a similar excess of an unlabeled oligonucleotide containing an NFκB binding site (lane 4). The specificity of the detected complex was further confirmed by its super-shift in the presence of anti-HSF1 antibody (lane 7). (B) QC does not affect nuclear HSF1 DNA binding activity induced by proteotoxic stress. EMSA was performed as in (A) with a labeled HSF1-specific oligonucleotide probe and nuclear extracts from HeLa cells left untreated (lane 1), or treated for 4 h with 5 μM MG132 (MG) or 1 μM 17-DMAG (GM) alone or in combination with 20 μM QC. (C) Bafilomycin increases the nuclear concentration of QC. HeLa cells were treated with 5 μM QC alone or in combination with 0.5 μM of bafilomycin for 1 h. The intracellular localization of QC was analyzed by UV-microscopy with a blue filter. (D) Bafilomycin increases the HSR inhibitory activity of QC. HeLa cells were left untreated (lane 1) or treated for 5 h with the indicated combinations of 5 μM MG132 (MG), 0.5 μM bafilomycin (Baf), and variable amounts of QC. Whole cell protein extracts were analyzed by western blotting with anti-hsp70 antibody. pirin was examined as a protein loading control (L.c.).
