Fig. 2.
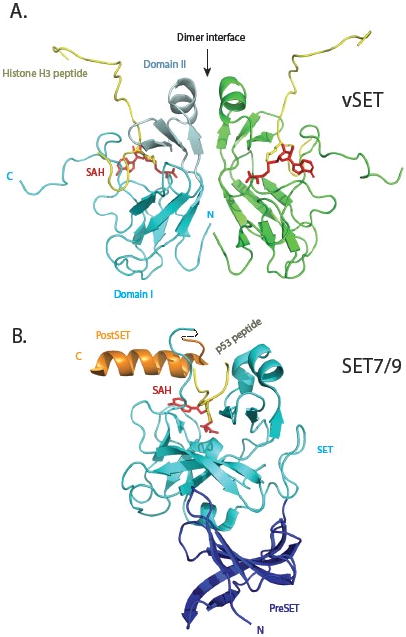
Comparison of three-dimensional structures of vSET and mammalian SET7/9 lysine methyltransferases. (A) Solution structure of vSET bound to cofactor SAH and histone H3 peptide containing mono-methylated lysine 27. The two protomers of the C2-symmetric dimer are shown in cyan and green. Domain I and domain II of one protomer are depicted in regular and pale cyan, respectively. The histone peptides are shown in gold, and SAH molecules in red. The N- and C-termini of the cyan protomer are labeled. (B) Crystal structure of SET7/9 in complex with p53 peptide and SAH. The preSET, SET and postSET domains are colored blue, cyan and orange, respectively. The peptide and cofactor are shown in the same colors as those in A.
