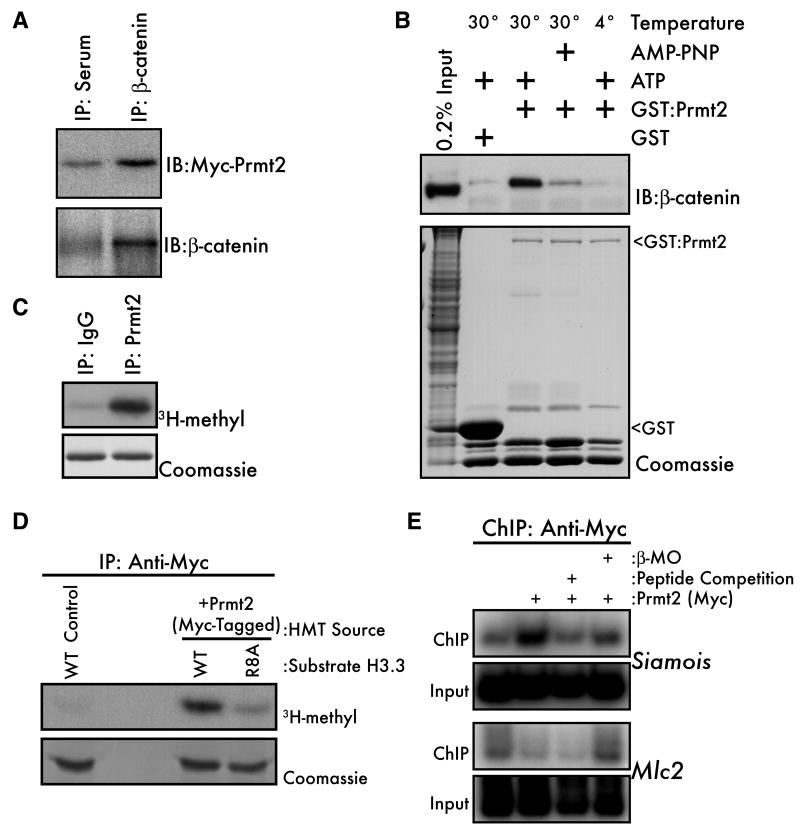
Figure 4. β-catenin interacts with the Histone H3(R8) methyltransferase Prmt2.
(A) Myc-tagged mouse Prmt2 (500pg) was expressed in Xenopus embryos and embryo lysates (early blastula) were immunoprecipitated with anti-β-catenin or pre-immune serum and subjected to western blot with either anti-myc (upper panel) or anti-β-catenin antibodies. (B) PreMBT embryo lysates were incubated for 1 hour with GST-Prmt2 beads at 4° or 30°C and with ATP or the nonhydrolyzable ATP analog AMP-PNP. Bound proteins were eluted and β-catenin was visualized by western blot (upper panel). GST beads alone were used as a negative control and the relative amounts of bait proteins in each lane were visualized by coomassie staining (lower panel, arrowheads). “Input” indicates non-fractionated input embryo lysate. (C) Endogenous Prmt2 from mouse embryonic stem cells was subjected to an IP/HMT assay using recombinant H3.3 as a substrate. Rabbit IgG was the negative control. (D) Activity of Myc-Prmt2 from 32- to 128-cell Xenopus embryos was measured by IP/HMT assay using wild-type (WT) or R8A histone H3.3 as substrates. Non-injected embryos served as negative controls. (E) Wild type or β-MO injected 1000-cell embryos expressing Myc-Prmt2 were subjected to ChIP using the anti-Myc-tag antibody. Non-injected embryos served as a negative control. The specificity of ChIP was confirmed by including a 200-fold excess of the Myc-peptide (lane 3) in the IP.