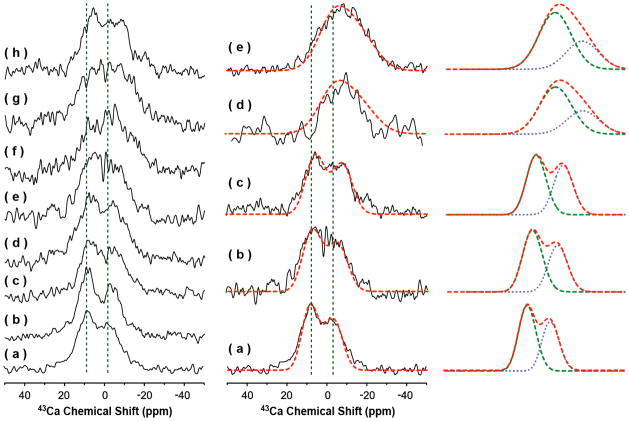
Figure 1. Natural-abundance 43Ca NMR spectra of powder samples of bone and model compounds.
First column: Natural-abundance 43Ca NMR spectra of (a) 0%, (b) 0.3%, (c) 2.3%, (d) 3.5%, (e) 5.0%, (f) 6.9%, (g) 8.8%, and (h) 10% carbonated apatite samples. Second column: Natural-abundance 43Ca NMR spectra of (a) hydroxyapatite, (b) 5% carbonated apatite, (c) 10% carbonated apatite, (d) deproteined cortical bone, and (e) cortical bone samples. Spectra were obtained on a 833 MHz solid-state NMR spectrometer using a single-resonance 4 mm MAS probe with a spinning frequency of 10 kHz at room temperature. All spectra were recorded using a spin-echo, 90°-τ-180°-τ-acquire, sequence with a 2 μs 90° pulse width, τ of 40 μs, and a 0.5 s recycle delay. 131,072 scans were used for figures a–d, while 480,000 and 262,144 scans were used for the deproteined bone (d) and cortical bone (e) spectra, respectively. A saturated solution of CaCl2 (at 0 ppm) was used as a standard to reference 43Ca chemical shift. The simulated spectra are displayed in red dashed lines (second and third columns). The simulated Ca-II (green) and Ca-I (blue) are also plotted for a comparison (in the third column).