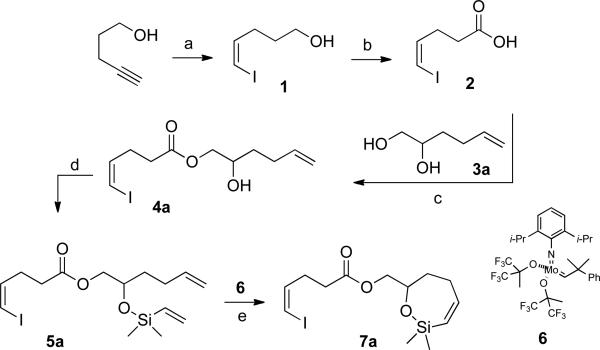
Scheme 1a.
a Conditions: (a) 1. I2, KOH, MeOH; 2. KO2CN=NCO2K, AcOH, THF/i-PrOH, (62%); (b) CrO3/H5IO5, MeCN/H2O (9/1), (97%); (c) 1. COCl2, benzene; 2. 3a, collidine, CH2Cl2, –78 °C, (79%); (d) chlorodimethylvinylsilane, Et3N, CH2Cl2 (93%); (e) 6, benzene, rt (87%). The yields are of analytically pure material.