Table 1.
Optimization of the Fluoride Source and Solvent for the Macrocyclizationa
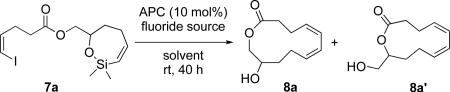 | |||||
|---|---|---|---|---|---|
| entry | fluoride source | equiv | solvent | yield, % 8a+8a' | ratiob8a+8a' |
| 1 | TBAF•3H2O | 10 | THF | 0 | - |
| 2 | TBAF•3H2O | 10 | THF | 43 | 3:1 |
| 3 | TBAF•4H2O | 10 | THF | 64 | 3:1 |
| 4 | TBAF•6H2O | 10 | THF | 59 | 3:1 |
| 5 | TBAF•6H2O | 5 | THF | 72 | 3:1 |
| 6 | TBAF•6H2O | 2.5 | THF | 26 | 3:1 |
| 7 | TBAF•6H2O | 5 | dioxane | 76 | 3:1 |
| 8 | TBAF•6H2O | 5 | DMF | 84 | 2:1 |
| 9 | TBAF•7H2O | 5 | DMF | 79 | 2:1 |
| 10 | TBAF•10H2O | 5 | THF | 46 | 100:0 |
| 11 | TBAF•15H2O | 5 | THF | 39 | 100:0 |
Reaction conditions employed 0.25 mmol of 7a at 0.1 M.
Ratio determined by 1H NMR analysis.
