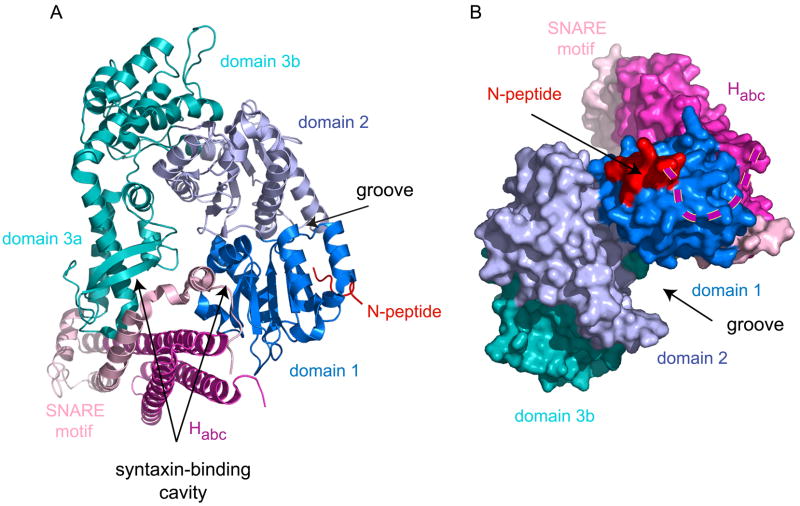
Figure 1. Structure of Munc18-1 bound to synaxin-1.
(A) A ribbon diagram illustrates the architecture of the conserved three-domain SM protein, Munc18-1, bound to the helical bundle of synaxin-1 in the closed conformation. Munc18-1 domain 1 (blue) binds the syntaxin N-peptide (red) and forms an arch with domains 2 (light blue) and 3 (teal). The cavity of the arch binds the syntaxin-1 helical bundle formed by the SNARE motif helix (light pink) bound to the Habc domain (hot pink) in the closed conformation. The location of the groove between domains 1 and 2 and the syntaxin-binding cavity are indicated with arrows.
(B) Rotated view reveals two syntaxin-binding sites and a groove implicated in SNARE complex binding. SM protein Munc18-1 [domains colored as in (A)] is viewed from the membrane end of the closed, 4-helix bundle of synaxin-1 [colored as in (A)]. The syntaxin N-peptide is bound to domain 1 of Munc18-1, and is connected to the syntaxin-1 Habc helical bundle by an unresolved linker (aa 10–26; hatched line). Mutations in the groove region of a yeast SM protein, Sec1p, abolish SNARE complex binding. The syntaxin-binding cavity and domain 3a are tilted into the page and are, therefore, not visible in this view. Images were created using Pymol™ (DeLano Scientific, Palo Alto, CA) and Protein Data Bank accession number 3C98.