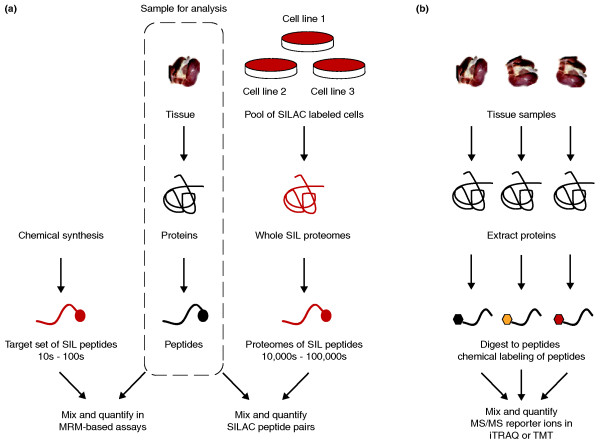
Figure 1.
Quantitative approaches in profiling complex tissue proteomes. (a) Quantification using exogenous stable isotope labeled (SIL) peptide standards. The sample to be analyzed is common to both forks in the workflow and is marked in the dotted box. Tissue samples are processed to extract proteins and digested with trypsin to generate complex mixtures of peptides. In a targeted MRM-based assay (left) [6,7], known amounts of chemically synthesized SIL peptides matching peptides from target proteins are introduced to the sample and serve as relative internal standards in peptide quantification. In an alternative workflow (right), pools of SILAC-labeled cells are combined; extracted proteins are digested with the same enzyme (trypsin) to generate a whole-proteome SIL peptide standard containing tens of thousands to hundreds of thousands of peptides [4]. This SIL proteome standard can be adjusted to match the cellular characteristics of the sample to be quantified. A large stock of a suitable proteome standard could be a common internal reference spiked into hundreds of experiments. (b) Quantification by derivatizing peptides with chemical labeling reagents. This is currently the most common approach for SIL-based quantification of whole-tissue proteomes. Peptides are tagged with chemical labels directed to specific functional groups, such as primary amines of the amino terminus and lysine residues. Commercially available reagents such as iTRAQ and TMT allow multiplexing of samples (up to eight with iTRAQ), but this may be a limiting factor if larger studies are desired.