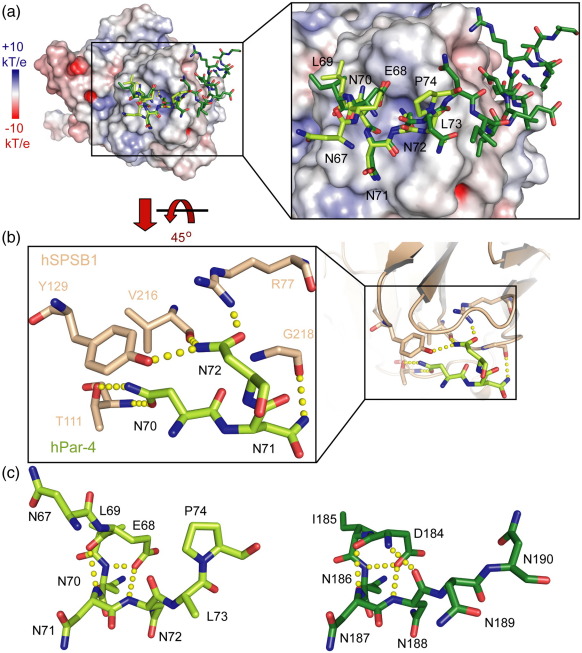
Fig. 4.
Comparison of the VASA and hPar-4 binding modes to hSPSB1. (a) Overlay of the hSPSB1 structures in complex with VASA (dark green) and hPar-4 (light green). The surface of hSPSB1 is colored by electrostatic surface potential. The peptide NNN motifs bind a shallow pocket centered on hSPSB1 Arg77. The peptide backbone deviates at hPar-4 Glu68 where the shorter Asp184 side chain of VASA enables an intramolecular main-chain hydrogen bond with VASA Asn188. (b) Stick representation showing the side-chain hydrogen bonds formed by the central hPar-4 NNN motif. Similar bonds are formed in the VASA complex. (c) Intramolecular hydrogen bonding in hPar-4 and VASA. The VASA conformation is stabilized by an additional main-chain hydrogen bond between Asp184 and Asn188.