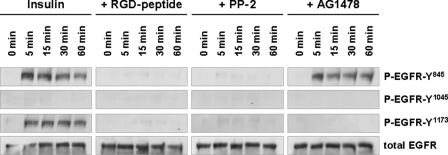
FIGURE 1.
Insulin-induced EGFR phosphorylation in perfused rat liver. Rat livers were perfused as described under “Experimental Procedures.” When indicated, RGD peptide (10 μmol/liter), PP-2 (250 nmol/liter), or AG1478 (1 μmol/liter) was added 30 min prior to the insulin institution (35 nmol/liter) to the perfusate to inhibit integrins, c-Src or EGFR tyrosine kinase activity, respectively. Liver samples were taken at the time points indicated, and phosphorylation of EGFR Tyr845, Tyr1045, and Tyr1173 was analyzed by use of phospho-specific antibodies. Total EGFR served as loading control. Representative Western blots of three independent perfusion experiments are shown. Within 5 min insulin induced an RGD peptide- and PP-2-sensitive phosphorylation of EGFR residues Tyr845 and Tyr1173, whereas no phosphorylation at position Tyr1045 is detectable within 60 min. AG1478 blunted insulin-induced Tyr1173 phosphorylation, whereas Tyr845 phosphorylation remained unchanged, suggestive of an EGFR Tyr845 transphosphorylation leading to EGFR Tyr1173 autophosphorylation.