FIGURE 7.
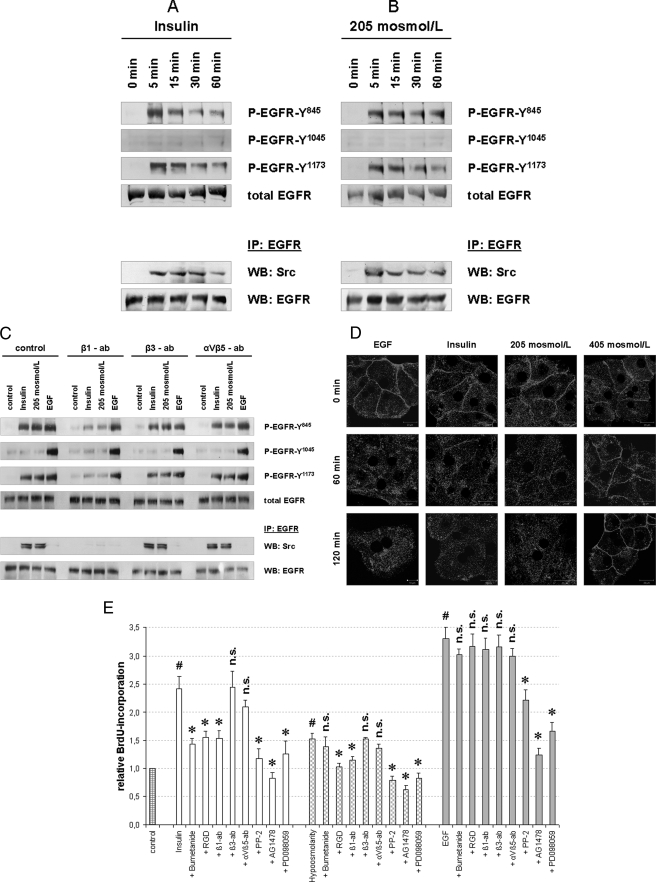
Insulin- and hypoosmolarity-induced EGFR activation and BrdU incorporation in primary rat hepatocytes. Primary rat hepatocytes were isolated as described under “Experimental Procedures” and seeded on collagen-coated wells for 48 h. Insulin- and hypoosmolarity-induced EGFR phosphorylation (A–C) and internalization (D) in primary hepatocytes are shown. Cells were incubated with insulin (100 nmol/liter), hypoosmolarity (205 mosmol/liter), or EGF (50 ng/ml) for the indicated time periods and then analyzed for EGFR phosphorylation at Tyr845, Tyr1015, and Tyr1173 by use of phospho-specific antibodies. Total EGFR served as loading control. In addition, EGFR was immunoprecipitated (IP) from the respective samples as described under “Experimental Procedures” and analyzed for EGFR/c-Src association by detection of c-Src in Western blots (WB). Total EGFR served as loading control. Representative Western blots of three independent experiments are shown. Insulin (A) and hypoosmolarity (B) induced EGFR phosphorylation at Tyr845 and Tyr1173, but not at Tyr1045, and EGFR/c-Src association. C, insulin-, hypoosmolarity- and EGF-induced EGFR phosphorylation and EGFR/c-Src association were tested for integrin blocking antibody sensitivity. Anti-integrin β1 subtype antibody (β1-ab, 1 μg/ml) inhibited insulin-induced (100 nmol/liter, 5 min) and hypoosmolarity-induced (205 mosmol/liter, 5 min) EGFR phosphorylation and EGFR/c-Src association, whereas anti-β3 (β3-ab, 1 μg/ml) and anti-αVβ5 subtype antibodies (αVβ5-ab, 1 μg/ml) were ineffective. As for control, EGF-induced (50 ng/ml, 5 min) EGFR phosphorylation was not affected by the used integrin-blocking antibodies. In another set of experiments, hepatocytes were cultured on glass coverslips and immunostained for EGFR expression as described under “Experimental Procedures.” EGFR localization was visualized by confocal laser scanning microscopy. D, insulin (100 nmol/liter) and hypoosmolarity (205 mosmol/liter) induced an EGFR internalization comparable with that induced by EGF (34, 39), whereas hyperosmolarity (405 mosmol/liter) induced EGFR enrichment at the plasma membrane (39). E, insulin- and hypoosmolarity-induced proliferation of primary hepatocytes is shown. After 48 h of cell culture, culture medium was removed and replaced by culture medium containing BrdU. Then, hepatocytes were stimulated for another 48 h with normoosmotic control medium (305 mosmol/liter), insulin (100 nmol/liter), hypoosmolarity (205 mosmol/liter), or EGF (50 ng/ml) and then analyzed for BrdU uptake. When indicated, bumetanide (10 μmol/liter), RGD peptide (100 μmol/liter), PP-2 (10 μmol/liter), AG1478 (5 μmol/liter), or PD098059 (10 μmol/liter) was instituted 30 min prior to insulin, hypoosmolarity, or EGF incubation. In addition, integrin blocking antibodies, i.e. anti-β1, anti-β3, and anti-αVβ5 antibodies (each 1 μg/ml), respectively, were used to inhibit the respective integrin subtypes. BrdU uptake in hepatocytes kept in normoosmotic control medium was arbitrarily set to 1. Statistical analyses of at least five independent experiments for each condition are shown. No significant inhibition compared with insulin, hypoosmolarity, or EGF incubation is indicated by n.s. (p > 0.05). Insulin, hypoosmolarity, and EGF induced a significant increase in hepatocyte proliferation by means of BrdU uptake (#, p < 0.05). Insulin- and hypoosmotic-induced hepatocyte proliferation was significantly inhibited (*, p < 0.05) by RGD, anti-integrin β1, PP-2, AG1478, and PD098059. Because the insulin-induced proliferation was also bumetanide-sensitive, these data suggest a swelling-dependent, integrin β1-, s-Src-, EGFR- and ERK-mediated hepatocyte proliferation upon either insulin or hypoosmotic stimulation. Error bars, S.E. of the mean.