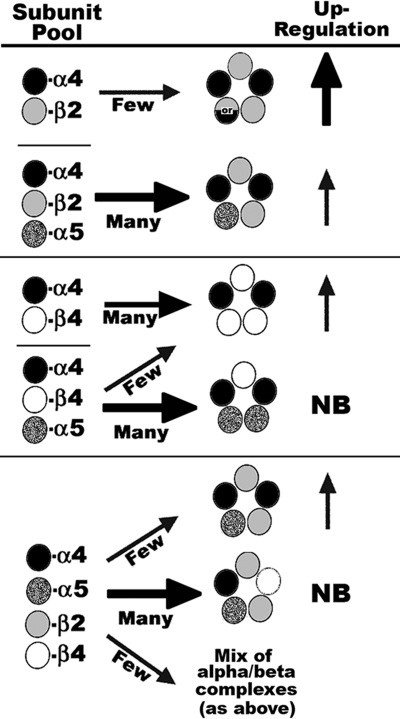
FIGURE 6.
Model of α5 impact upon assembly of nAChRs. Depending upon the β subunit (either β2 or β4), the relative outcome of assembly with α4 varies dramatically when α5 is included in the mixture. Although α4+β2 does assemble to bind ligand with high affinity (as indicated by upward arrows), α5 favors this assembly through possibly filling the fifth position in nAChR assembly. The relative level of up-regulation is indicated by the size and thickness of the arrow. Two possible stoichiometries for α4+β2 receptors that were not investigated in this study are designated by the mixed circle containing or (31). In α4+β4 mixtures, α5 competes with β4 for binding to α4, resulting in reduced complexes detected by ligand binding. Although assembly of α4+β2 containing nAChRs is favored by α5, this decreases the relative amount of up-regulation promoted by nicotine because this subunit takes the place of nicotine in stabilizing or promoting expression of maturing nAChR complexes. The majority of complexes formed from co-transfection of α4+α5+β2+β4 result in mostly subunit associations that are not detected by ligand binding. NB, no binding. However, these mixtures also give rise to α4+β4±α5 complexes and α4+α5+β2 complexes, which dominate ligand binding measurements.