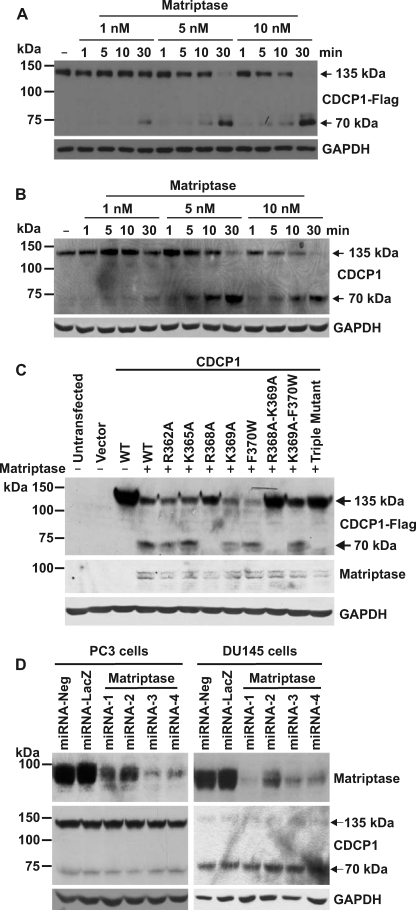
FIGURE 6.
The serine protease matriptase is an efficient but not essential processor of CDCP1. A, anti-Flag Western blot analysis of lysates from HeLa-CDCP1-Flag cells either untreated (−) or incubated with 1, 5, or 10 nm matriptase for 1, 5, 10 or 30 min. B, Western blot analysis of lysates from 22Rv1 cells either untreated (−) or incubated with 1, 5, or 10 nm matriptase for 1, 5, 10, or 30 min using a goat anti-CDCP1 antibody (Abcam ab1377). Anti-GAPDH Western blot analysis was performed to examine protein loading. C, HeLa cells were either untransfected, transfected with vector alone, or co-transfected with matriptase and either wild type (WT) CDCP1-Flag expression construct or constructs encoding the indicated single and double residue mutant CDCP1-Flag constructs or triple mutant CDCP1-Flag-R368A-K369A-F370W. After 24 h, lysates were collected and analyzed by anti-Flag, anti-matriptase, and anti-GAPDH Western blot analysis. D, polyclonal populations of PC3 and DU145 cells were stably transfected with one of four CDCP1 knockdown miRNA constructs. After 4 weeks of selection in blasticidin, cell lysates were analyzed by Western blot analysis using rabbit anti-matriptase, goat anti-CDCP1 (Abcam ab1377), and anti-GAPDH antibodies.