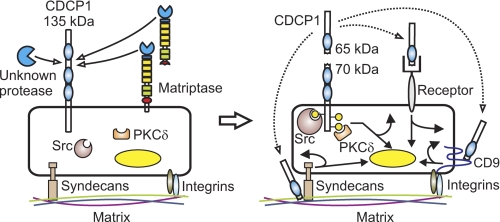
FIGURE 9.
Protease-mediated processing of CDCP1. Serine proteolysis generates a 65 kDa shed ectodomain and a tyrosine-phosphorylated cell retained 70 kDa CDCP1 fragment. Proteolysis occurs at Arg-368 and Lys-369 between CUB-like domain 1 and 2 of CDCP1 (the 3 CDCP1 CUB-like domains are shown as blue ovals). Proteolysis results in tyrosine phosphorylation of 70 kDa CDCP1 (yellow-filled circles) and recruitment of Src and PKCδ in a phosphorylation-dependent manner. We propose that the CDCP1 ectodomain may function as a ligand or competitive inhibitor for a cell surface receptor (autocrine receptor binding is shown, but paracrine and endocrine signaling may also be relevant) or as a matrix-interacting protein potentially modulating cell:matrix interactions occurring via known CDCP1 interacting proteins such as syndecan 1 and 4 and the tetraspannin CD9 (dotted arrows). Potential signaling downstream of the 70 kDa CDCP1 cell-retained fragment and ectodomain resulting in direct cellular responses and changes in gene expression are represented by curved and straight arrows, respectively. Currently the endogenous serine protease or proteases mediating cleavage of CDCP1 at Arg-368 and Lys-369 are not known. However, we have shown that the cell surface and shed serine protease matriptase efficiently cleaves CDCP1 exclusively at Arg-368.