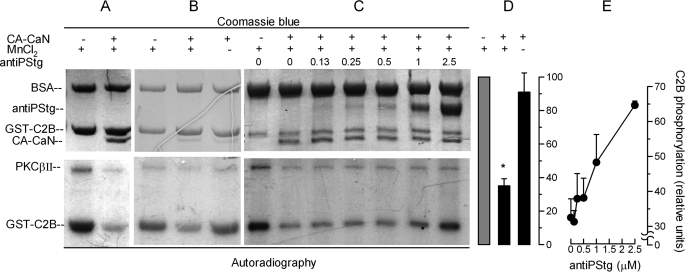
FIGURE 6.
Synaptotagmin is a substrate for calcineurin. A–C, GST-C2B domains immobilized in glutathione-Sepharose beads were incubated for 40 min at 37 °C with PKCβII under activating conditions in the presence of [γ-32P]ATP. The beads were washed and then incubated for 1 h at 30 °C in the presence or absence of the constitutively active catalytic domain of calcineurin (CA-CaN) in a buffer containing 1 mm MnCl2 (MnCl2 +) or 1 mm EGTA (MnCl2 −). In some experiments, increasing concentrations of an antibody that recognizes the phosphorylated polybasic region of the C2B synaptotagmin domain were added (antiPStg, 0–2.5 μm). The samples were then resolved by SDS-PAGE. Total proteins (top panels) are shown by Coomassie Blue stain. Phosphorylated proteins were detected by autoradiography (bottom panels). D, autoradiographies from three experiments as shown in B were quantified and normalized for the protein load. The data represent the means ± S.E. The asterisk indicates a significant difference from incubation without CA-CaN (one-way ANOVA and 99% confidence interval). E, autoradiographies from two experiments as shown in C were quantified. The data represent the means ± range.