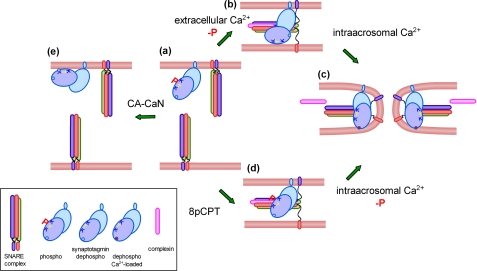
FIGURE 8.
Working model for the Ca2+/calcineurin-dependent dephosphorylation of synaptotagmin during acrosomal exocytosis. In resting sperm, SNAREs are assembled in inactive cis-complexes, and synaptotagmin is phosphorylated (a). Upon activation, Ca2+ coming from the extracellular medium triggers SNARE complex disassembly and calcineurin-dependent synaptotagmin dephosphorylation. Acrosomal swelling and deformation of the granule membrane favor the tethering with the plasma membrane. SNAREs can then reassemble in trans in combination with complexin; dephosphorylated synaptotagmin is incorporated into the prefusion complex probably interacting with phospholipids in the opposite membrane through the polybasic region (b). We speculate that the calcium concentration at this stage is not sufficient to insert the aspartic-rich Ca2+-binding loops of synaptotagmin into the membrane and to relieve the complexin clamp. These events must wait for the local influx of calcium coming from the acrosome to promote full SNARE complex assembly and membrane fusion (c). When sperm are activated at low calcium concentrations (e.g. with the cAMP analog 8pCPT), the system progresses to the stage where loose trans-complexes are assembled, but synaptotagmin remains phosphorylated (d). The influx of calcium from the acrosome is sufficient to dephosphorylated synaptotagmin at this stage, and the membrane fusion process is completed (c). Unexpectedly, when constitutively active calcineurin is added to resting sperm at low calcium concentrations, synaptotagmin is dephosphorylated, and exocytosis is inhibited (e). The protein may be engaged in unproductive cis-interactions that prevent exocytosis.