FIGURE 1.
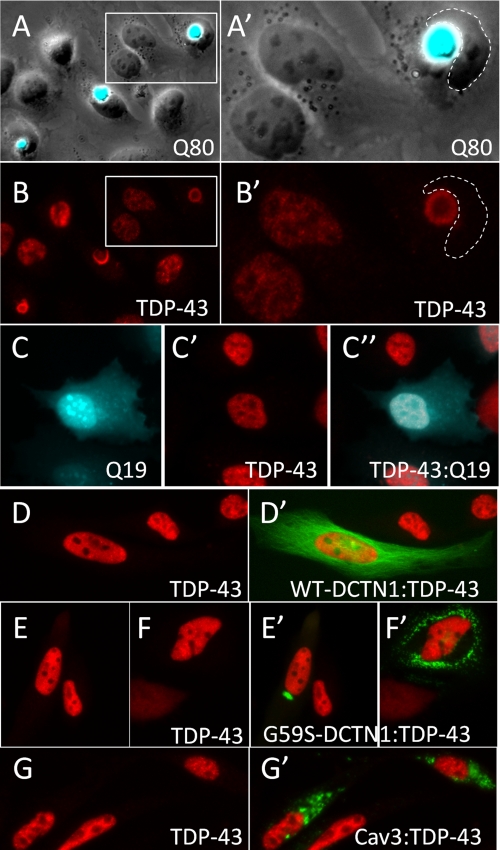
Cytoplasmic polyglutamine aggregates bind and sequester nuclear TDP-43. A, HeLa cells expressing an expanded polyglutamine construct Q80-CFP developed large cytoplasmic polyglutamine aggregates (A, overlay of phase contrast and CFP fluorescence images). B, immunofluorescence staining to visualize TDP-43 in cells with Q80-CFP aggregates showed that endogenous TDP-43 was completely sequestered into the Q80 aggregate and was absent from the nucleus. A′ and B′ represent higher magnification images of the boxed regions shown in A and B. Cells transfected with Q19-CFP (C) showed normal nuclear localization of endogenous TDP-43 (C′, TDP-43, C″- overlay). d–G, TDP-43 immunostaining in HeLa cells transfected other aggregation prone proteins. Wild-type dynactin-1(WT-DCTN1) fused to GFP (D′) showed normal distribution along microtubules, whereas the G59S-DCTN1 mutant formed discrete focal or multifocal ubiquitinated cytoplasmic aggregates (E′ and F′). G, GFP-Caveolin-3 with the P104L point mutation (Cav3) also formed cytoplasmic ubiquitinated aggregates when transfected into HeLa cells. Unlike Q80 polyglutamine aggregates, cytoplasmic aggregates of G59S-DCTN1 (E′ and F′) and Cav3 (G′) did not induce translocation to the cytosol or sequestration of endogenous TDP-43.