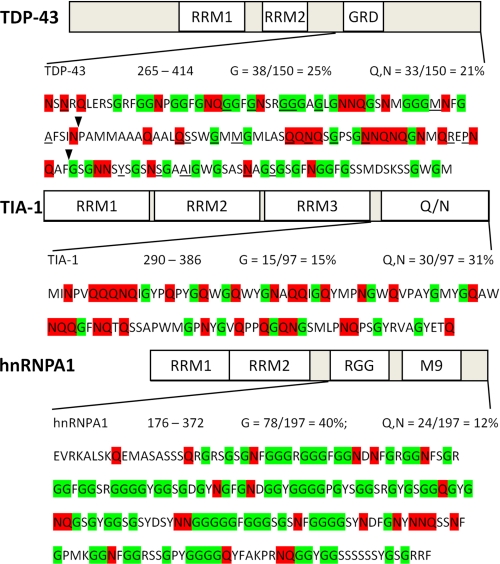
FIGURE 4.
Sequence of the C-terminal domains of TDP-43, TIA-1, and hnRNPA1 reveals a Q/N-rich domain in TDP-43. TDP-43 is shown at the top, followed by TIA-1, which contains a well characterized Q/N-rich domain at the C terminus, and hnRNPA1, which contains a canonical glycine-rich domain of the 2×RBD-Gly family, composed of an RGG domain and an M9 nuclear shuttling signal. Glycine (G) residues are highlighted in green and glutamine (Q) and asparagine (N) residues are in red. The overall Q/N content of the C terminus of TDP-43 is 21%. The core region required for the interaction of TDP-43 with polyglutamine aggregates identified in the deletion analysis (flanked by arrowheads; 320–367) shows 31% Q/N content, similar to the Q/N-rich prion-related domain of TIA-1. By contrast, the C-terminal region of hnRNPA1 is significantly more glycine-rich than TDP-43 but has low Q/N content and does not bind polyglutamine aggregates. All but one of the currently described mutations in TDP-43 (underlined) are located in the C-terminal domain.