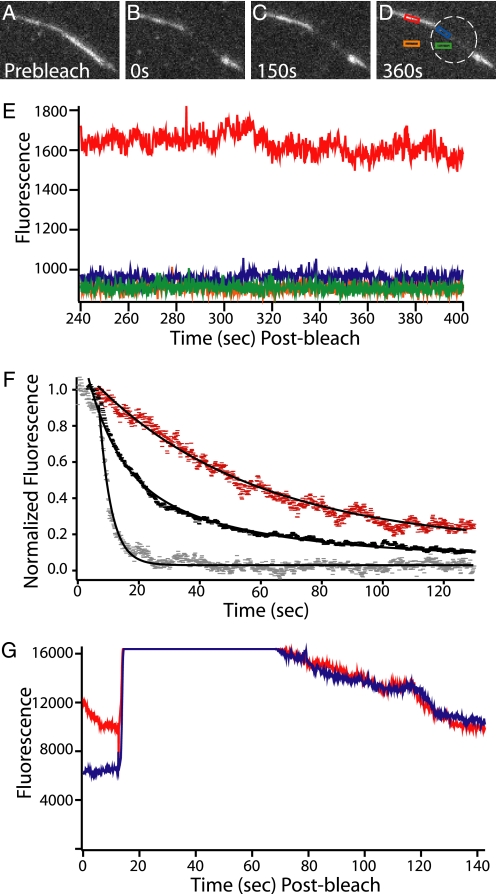
FIGURE 5.
Fluorescence decay and FRAP of labeled fascin in bundles shows the fascin population is stable unless a competitor is added. A–D, bundle of dark actin held together by fluorescent atto-647-fascin (labeled on exposed cysteines using maleimide chemistry, measured 0.95 dyes/fascin) is bleached and then observed in a series of movies over 400 s. Before bleaching, the flow cell was rinsed with buffer to remove free fascin from solution. The boundary between the bleached and unbleached regions of the bundle remained sharp and the signal from the unbleached portion of the bundle remained nearly constant. The white ring indicates the zone of bleaching. Low observational laser power was used to facilitate the long acquisition time by minimizing photobleaching. E, fascin in the bundle is stable in the absence of cross-linker in solution. The graph shows the fluorescence decay profile of the four highlighted regions from D, recorded during the final movie of this observation. The fluorescence of the bundle (red) remains stable and higher than the other three regions. The green curve is background in the bleached zone; orange is the background outside of the bleached zone, and blue is the bundle in the bleached zone. F, fascin in bundles can be competed away. A series of buffer wash experiments using bundles similar to those in A–D were performed. In all cases the bundles had been rinsed with buffer to remove free fascin from solution before data were recorded. All data points are background subtracted and then normalized so that a value of 1 corresponded to the average value of the first 25 data points after the wash was initiated. When the bundles were washed with buffer only (red), a slow decay (0.019 ± 0.003 s−1, S.E.) was seen in the fluorescent signal. This corresponds to the photobleaching rate at the laser powers used in these experiments. When 3 μm unlabeled fascin (black) was washed in a double exponential decay was observed. The faster rate corresponds to fascin being displaced from the bundle (0.10 ± 0.007 s−1, S.E.). This value closely matches that of Aratyn et al. (38), who reported a decay rate of 0.12 s−1. The slower rate (0.023 ± 0.002 s−1, S.E.) corresponds to photobleaching. When 3 μm unlabeled α-actinin was washed in a rapid single exponential decay was observed (0.254 ± 0.004 s−1, S.E.). This is even more rapid than the decay observed with the addition of fascin. This shows that the presence of a competitive agent causes rapid cross-linker turnover. G, fascin replacement is uniform and complete across the entire bundle. With no fascin in solution, a region of a bundle made with fluorescent fascin was bleached. Blue indicates the area of the bundle that was bleached, and red indicates the area that was not bleached. After bleaching, a significant difference in signal between the two curves is observed. Next, 3 μm fluorescent fascin was washed into the chamber (around the 20-s mark, where signal rises to saturation). It was allowed to incubate for 30 s and then was washed out with buffer. The wash is completed, and the signal drops to resolvable levels after approximately an additional 20 s. At this point, the bundle inside and outside the bleach zone have the same fluorescence. This confirms that free fascin can incorporate completely and evenly into the bundle.