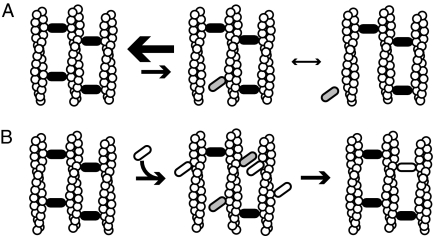
FIGURE 6.
Models of bundle formation and dynamics. A, bundles are stable in the absence of competitive agents. Cross-linking proteins that are bound to two filaments (black ovals) stabilize filament bundles. These proteins can toggle to a state (gray ovals) where they are only bound to a single filament. Because the actin site is restrained, the single bound cross-linker can readily rebind the second filament. This rebinding rate is faster than the dissociation rate in the single bound state, yielding bundles that are very stable. B, when a competitive agent (white oval) is added to a stable bundle it can occupy actin sites near single bound cross-linkers, preventing their rebinding. This leads to dissociation of the endogenous cross-linker and either replacement with the exogenous agent (shown) or dissociation of both factors (not shown). If cross-linking protein is abundant in solution, there is a constant exchange of proteins in the bundle as shown by previous FRAP experiments.