FIGURE 6.
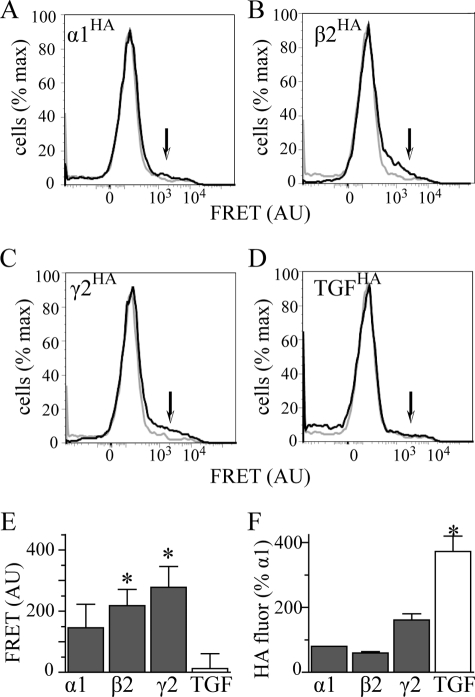
Interactions of the α1(AD) subunit with wild type GABAAR subunits. We transfected HEK293T cells with 2 μg of α1(AD)h subunit and 0.125:0.250:0.250 μg ratios of either α1HAβ2γ2 (A), α1rβ2HAγ2 (B), or α1rβ2γ2HA subunits to form receptors in which either the α1, β2, or γ2 subunit is tagged with the HA epitope. In addition, to determine whether the α1(AD)h subunit interacted nonspecifically with the TGFHA protein, we transfected cells with 2 μg of α1(AD)h subunit, a 0.125:0.250:0.250 μg ratio of α1rβ2γ2 subunits, and 0.250 μg of TGFHA (D). We permeabilized the cells and stained them with either the anti-α1h-647 antibody (FRET acceptor), the antiHA-555 antibody (FRET donor), or both anti-α1h-647 and antiHA-555 antibodies. A–D are flow cytometry histograms of FRET fluorescence. The gray line plots the histogram for cells stained with only the α1h-647 acceptor antibody, and the black line plots the histogram for cells stained with both HA-555 donor and α1h-647 acceptor antibodies. The arrows point to the regions of the histograms where one would find specific FRET fluorescence. We quantified the specific FRET fluorescence and plotted it in E (n ≥ 5). Samples transfected with α1β2HAγ2 and α1β2γ2HA receptors possessed substantial FRET fluorescence that significantly differed compared with samples transfected with TGFHA protein. This difference in FRET fluorescence did not result from reduced HA fluorescence from the TGFHA protein because TGFHA possessed substantially more HA florescence than HA-tagged GABAAR subunits (F). AU, arbitrary units.