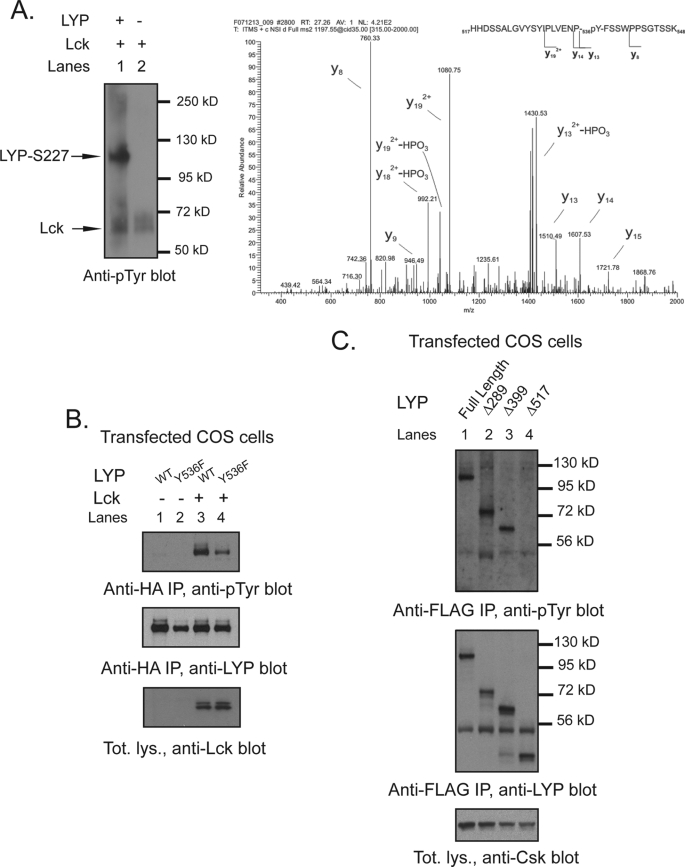
FIGURE 7.
Tyr536 is a direct phosphorylation site for Lck but not for Csk. A, detection of Tyr(P)536 by phospho-mass spectrometry. Recombinant LYP-S227 was in vitro phosphorylated with Lck, and the resulting protein mixture was separated by SDS-PAGE and stained by Coomassie. The LYP protein band was excised and digested in gel by trypsin. The resulting peptide mixture was analyzed by nanoLC-MS/MS. The panel shows the MS/MS spectrum of the peptide spanning residues 517–548 of LYP, showing fragment ions that unambiguously pinpoint phosphorylation of residue Tyr536. B, Tyr536 is a major Lck phosphorylation site. Anti-HA IPs were performed from lysates of COS cells transfected with LYP-WT and LYP-F536 alone (lanes 1 and 2) or with Lck (lanes 3 and 4). C, Tyr536 is an unlikely Csk phosphorylation site. Anti-FLAG IPs were performed from lysates of COS cells transfected with Csk together with FLAG-tagged full-length LYP-S227 (lane 1) or the truncation mutants Δ288LYP (lane 2), Δ399LYP (lane 3), and Δ517LYP (lane 4).