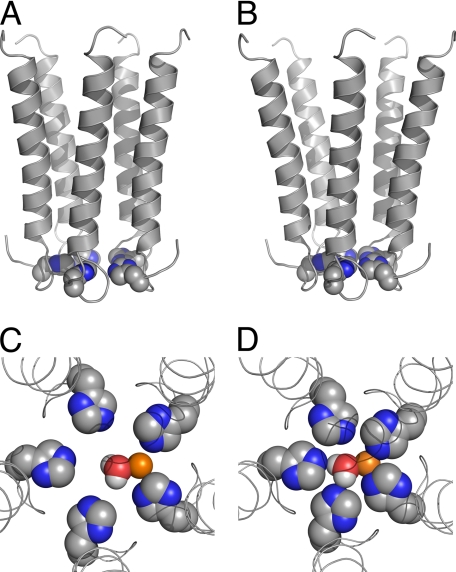
FIGURE 9.
Putative Zn2+ ion coordination within the ion channel pore. A and B, side views of the five pore-lining helices (M2s) of the putative active (A) and deactivated (B) channel conformations of chimera H-5′. C and D, top views of the putative activation/deactivation gate of chimera H-5′, which consists of five histidines organized around the axis of ion permeation pathway as a ring that accommodates a Zn2+ ion with an equatorially bound water molecule; shown are the putative active (C) and deactivated (D) gate conformations. Space-filling model of the histidines, Zn2+, and water are shown with the following colors: carbon, gray; nitrogen, blue; oxygen, red; hydrogen, white; Zn2+, orange. In the open pore model (C), the center-to-center distance between docked Zn2+ and the Nϵ atoms of the imidazole groups is 3.2 Å, whereas in the closed pore model (D) it is 2.3–2.4 Å. In both models the water oxygen is 2.3 Å away from the Zn2+ ion (center-to-center). Note that in water a Zn2+ ion is surrounded by a first hydration shell of six water molecules (87), so additional water molecules are probably bound to the Zn2+ ion above and below the shown plane. Notably, the models depicted here probably represent one of several possible different conformations that histidines may adopt, whereas Zn2+ itself can presumably induce changes in the histidine conformation.