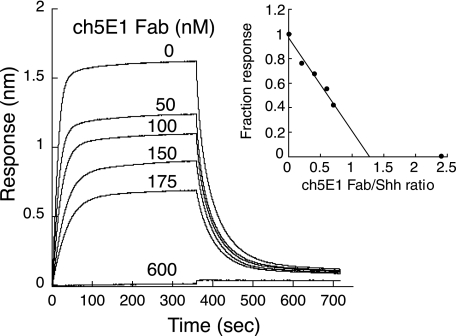
FIGURE 7.
Competition binding of ch5E1 Fab with Hhip L2 peptide binding to Shh. Biolayer interferometry sensorgrams of 250 nm Shh binding to biotinylated Hhip L2 peptide on streptavidin-coated biosensors are shown in the presence and absence of various concentrations of ch5E1 Fab. The inset shows the fraction response after binding at equilibrium versus the molar ratio of ch5E1 Fab/Shh. Because Shh and ch5E1 concentrations used are well above the KD value, ch5E1 effectively titrates Shh, having a molar ratio of 1.25 (x axis intercept), close to the predicted value of 1.0. At molar ratio 2.4, there is no observed binding of Shh to the Hhip L2 peptide.