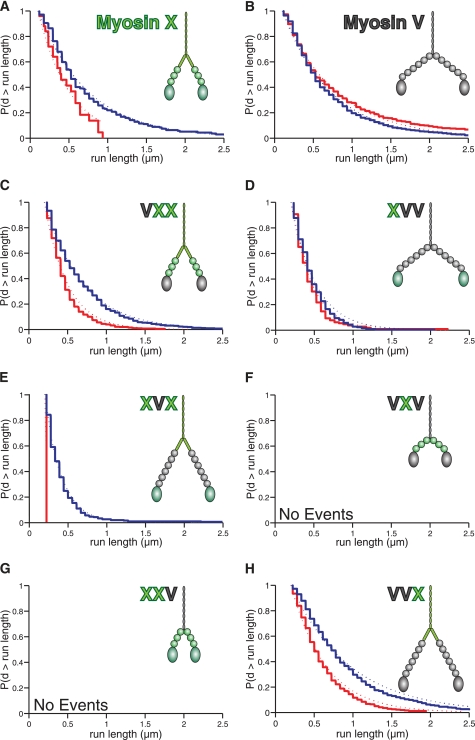
FIGURE 2.
Run lengths of chimeras reveal a key role for the myosin X tail in selecting bundles. The domain composition of each construct is shown for visual clarity of their size. The Kaplan-Meier estimate of the run length survivor function is shown for each processive construct on fascin-actin bundles (blue) and single actin filaments (red) at 2 mm ATP. Events are left-truncated at 0.2 μm and are right-censored at track ends. Run lengths are estimated from single exponential fits to the empirical survivor function (dotted lines). Run length decay constants (±S.E.) are: A, myosin X, filaments: 0.17 ± 0.05 μm (n = 24); bundles: 0.63 ± 0.08 μm (n = 100). Data are from Ref. 7. B, myosin V, filaments: 0.66 ± 0.05 μm (n = 231); bundles: 0.57 ± 0.06 μm (n = 134). Data are from Ref. 7. C, VXX, filaments: 0.29 ± 0.01 μm (n = 779); bundles: 0.49 ± 0.02 μm (n = 571). D, XVV, filaments: 0.27 ± 0.02 μm (n = 129); bundles: 0.31 ± 0.02 μm (n = 334). E, XVX, filaments: not determined (n = 2); bundles: 0.21 ± 0.01 μm (n = 323). F, VXV: not determined. G, XXV: not determined. H, VVX, filaments: 0.45 ± 0.02 μm (n = 556); bundles: 0.76 ± 0.02 μm (n = 817). The VXV and XXV chimeras were nonprocessive on both structures. Note the longer run lengths on bundles for the constructs containing the myosin X tail. The differences in run lengths are significant for VXX and VVX (p = 6 × 10−21 and 2 × 10−20, respectively; using the Kolmogorov-Smirnov test) and apparent for XVX, despite the low number of observed events on single filaments. The mean velocities measured in this single-molecule TIRF assay are (nm/s ± S.D.): VXX, filaments: 320 ± 100; bundles: 280 ± 90. XVV, filaments: 350 ± 110; bundles: 320 ± 110. XVX, bundles: 280 ± 100. VVX, filaments: 290 ± 110; bundles: 300 ± 120.