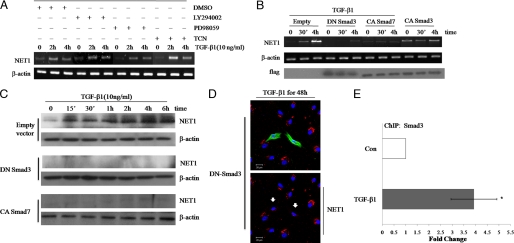
FIGURE 7.
Smad3 regulates RhoA activation through NET1. A, MEK, Akt, and phosphatidylinositol 3-kinase signaling do not affect TGF-β1-induced NET1 mRNA expression. Serum-starved ARPE-19 cells were pretreated for 1 h with vehicle (DMSO) or 10 μm MEK, Akt, or phosphatidylinositol 3-kinase inhibitors (PD98059, Triciribine (TCN), or LY294002, respectively) and then treated with 10 ng/ml TGF-β1 for 0, 2, or 4 h. After treatment, total RNA was isolated, reverse-transcribed, and amplified by PCR. β-Actin was evaluated as a loading control. B, Smad3 regulates NET1 expression. ARPE-19 cells were transfected for 12 h with plasmids expressing dominant-negative (DN) Smad3, constitutively active (CA) Smad3, or constitutively active Smad7. Empty vector was used as a control. After transfection, cells were switched to serum-free medium for 3 h and treated with TGF-β1 (10 ng/ml) for 0, 0.5, or 4 h. After treatment, total RNA was isolated, reverse-transcribed, and amplified by PCR and analyzed by Western blot using a FLAG-specific antibody. C, cells were treated as in B, lysed, and analyzed by Western blot using a NET1-specific antibody. D, Smad3 induces cytoplasmic localization of NET1. ARPE-19 cells were transfected with DNA plasmid and treated with TGF-β1 (10 ng/ml) for 48 h as described in B. Cells were fixed and stained with anti-FLAG followed by Alexa 488 (green) and stained with anti-NET1 followed by Alexa 546 (red). Blue is Hoechst nuclear staining. Pictures were taken under a Zeiss confocal microscope. Arrows indicate transfected cells. Bar, 20 μm. E, NET1 is a Smad3 target gene. ChIP analysis was performed using an anti-Smad3 antibody, and quantitative PCR was performed with primers corresponding to the NET1 promoter region. Cells were left untreated or stimulated with TGF-β1 for 30 min. Quantitative ChIP values are expressed as -fold change in site occupancy and represent the average and S.D. from three independent experiments. Con, control.