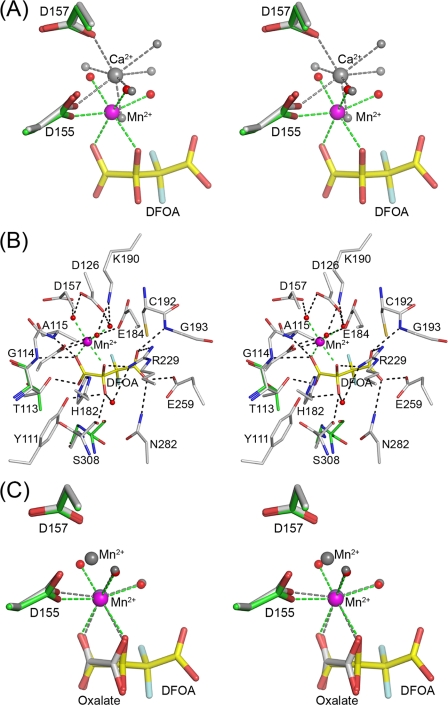
FIGURE 6.
Active site of OAH. A, stereoscopic representation of the metal coordination in the ligand-free and ligand-bound OAH structures. Atomic colors used are as follows: oxygen, red; fluorine, light blue; carbon, green (inhibitor-bound structure), gray (ligand-free structure; yellow, inhibitor. Mn2+ is depicted as a magenta sphere and Ca2+ as a gray sphere. Water molecules are represented by red spheres (the inhibitor-bounds structure) and gray spheres (ligand-free structure). Dashed lines show metal coordination. B, stereoscopic representation of the OAH active site containing the DFOA. Atomic colors used are as follows: carbon, gray for protein and yellow for inhibitor; oxygen, red; nitrogen, blue; fluorine, light blue; Mn2+, magenta. Also shown are two residues that adopt different conformations in the ligand-free structure; the backbone of Ser-308 is flipped, and the side chain of Thr-113 exhibits a different rotamer. The carbon atoms of these two residues are colored green. C, superposition of the metal ions and ligands in the OAH complexes bound with DFOA and oxalate. Color scheme as in A except that the gray colors are used to depict the Mn2+ and protein carbon atoms of the OAH·oxalate complex.