Abstract
Actinic keratoses are common intra-epidermal neoplasms that lie on a continuum with squamous cell carcinoma. Tightly linked to ultraviolet irradiation, they occur in areas of chronic sun exposure, and early treatment of these lesions may prevent their progression to invasive disease. A large variety of effective treatment modalities exist, and the optimal therapeutic choice is dependent on a variety of patient- and physician-associated variables. Many established and more recent approaches are discussed in this review with a focus on efficacy and administration techniques. Several previously experimental options, such as imiquimod and photodynamic therapy, have become incorporated as first-line options for the treatment of actinic keratoses, while combination treatment strategies have been gaining in popularity. The goal of all therapies is to ultimately limit the morbidity and mortality of squamous cell carcinoma. (J Clin Aesthetic Dermatol. 2009;2(7):43–48.)
Actinic keratoses (AKs) rank second among reasons to visit the dermatologist.1 Their incidence is tightly linked to ultraviolet (UV) irradiation, as they are found on chronically sun-exposed skin, occur among those with advancing age and lighter skin, and because sun protection reduces their incidence.2 AKs are thought by some to be precursor lesions to invasive squamous cell carcinoma (SCC), and by others as bona fide low-grade SCC in situ.3 Irrespective of this linguistical differentiation, AKs lie on a clinical, histological, and molecular continuum with SCC, and it is largely felt that early treatment of these lesions limits progression to invasive disease. Fortunately, a variety of effective treatments are available for the management of AKs. In general, the existence of such a wide assortment of treatment options for AKs is related to their superficial nature and amenability to rapid and efficient in-office or patient-administered treatment modalities. While more destructive therapies may have a high success rate, they also increase the chances for scarring or long-term pigmentary changes that would be unacceptable to many patients. A variety of non- to minimally invasive treatment strategies are readily available, and the decision to use certain approaches over others is based on myriad patient- and physician-associated factors, and frequently, a combination of schemes may be employed for optimal effect.
Should All Actinic Keratoses be Treated?
The diagnosis of AKs is primarily clinical, with the seasoned dermatologist having little difficulty recognizing their appearance. They emerge as ill-defined pink to skin-colored, scaly papules and small plaques on chronically sun-exposed areas of light-skinned individuals (Figure 1). They are most commonly located on the face, ears, balding scalp, extensor forearms, and dorsal hands. Studies have confirmed that histological diagnosis is in agreement with clinical suspicion more than 90 percent of the time, with only 1 in 25 lesions clinically consistent with AK revealing occult invasive disease.4
Figure 1.
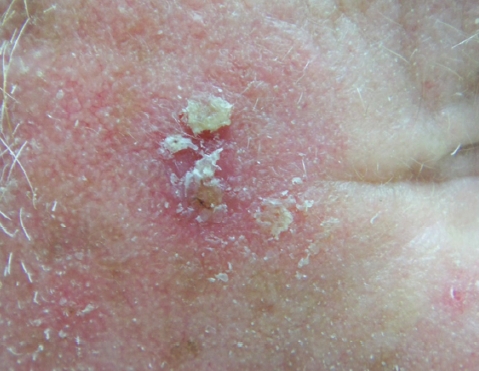
Actinic keratosis. Ill-defined pink scaly papule on the right temple of a fair-skinned individual.
Interestingly, while studies have shown a reduction in incidence of both AKs and SCC through sun protection, there has not been a randomized, controlled study demonstrating a decrease in SCC frequency with treatment of AKs. In practice, the relationship between AKs and SCC and the process of AK progression to invasive SCC are difficult to characterize precisely. Within the dermatological literature, reports of the frequency of AKs transforming to SCC differ by as much as three-fold.5 The precise number is difficult to calculate, as histological changes are similar for both AKs and SCC (e.g., cytologic atypia, dysplasia, cellular alteration), and lesions of AK are frequently found adjacent to SCC. Marks et al reported that 60 percent of SCC arise from AKs,6 while Huritz et al estimated this figure to be 97 percent.7 A yearly incidence rate of SCC of 0.24 percent among people with multiple AKs (average of 7.7 lesions) has been shown.8 Using these data, Dodsen et al mathematically determined that 6 to 10 percent of patients with multiple AKs will develop a SCC within a 10-year span, thus making it a reasonable endeavor to treat AKs with the goal of reducing morbidity, mortality, and cost associated with invasive SCC.9
Factors Contributing to Treatment Decision
Many factors are taken into account when determining which AK lesions to treat and how to treat them (Table 1). Warino et al recently presented a comprehensive report on the frequency of visits for AKs, methods used to treat them, and the annual costs of these treatments.10 The most effective strategy in the management of AKs is prevention through sun avoidance and diligent use of broad-spectrum sunscreens and blocking agents or protective clothing. Large, randomized studies have confirmed this notion by demonstrating a reduction in the development of new AKs and nonmelanoma skin cancers with UV radiation physical blockers and sunscreens.2,11 Nonetheless, with cumulative sun exposure and advancing age, rates of AK development increase, necessitating either ablative or topical treatment (Table 2).
Table 1.
Factors influencing treatment of actinic keratoses
| Cost |
| Reimbursement |
| Speed |
| Efficacy |
| Patient tolerability |
| Cosmesis |
| Number of lesions to treat |
| Spot vs. field treatment |
| Patient compliance |
| Patient history of multiple skin cancers |
| Immunosuppression |
| High-risk location (ears, lips) |
| Lesion morphology (hyperkeratotic, ill-defined) |
| Symptoms (pain, bleeding) |
| Previous treatment history |
Table 2.
Treatment modalities for actinic keratoses
| Prevention |
| Cryosurgery |
| 5-fluorouracil (5-FU) |
| Imiquimod |
| Photodynamic therapy (PDT) |
| Diclofenac |
| Adapalene |
| Curettage |
| Excision |
| Ablative Lasers |
| Chemical Peels |
| Dermabrasion |
Cryotherapy
Cryotherapy with liquid nitrogen is the established workhorse of AK treatment. By far, the most commonly employed therapeutic modality in the United States, it can be performed quickly and effectively in the office setting. Furthermore, as a physician-administered treatment with effectiveness related to freezing time, it can be tailored over a broad range to specific responses elicited in the skin. Drawbacks to this approach are related to patient discomfort, the inability to treat large areas, and the risk of residual hypopigmentation. In the largest prospective study investigating the use of cryosurgery for AKs, the overall complete response rate was 67.2 percent, varying from 39 percent for freeze times less than five seconds to 83 percent for freeze times longer than 20 seconds. The authors also reported that hypopigmentation resulted in 29 percent of the areas treated and was directly correlated with freezing times.12
Topical treatments for AKs can reduce the drawbacks of cryotherapy related to hypopigmentation; however, they require prolonged use and their success is, in part, determined by patient compliance. The greatest advantage of topical approaches lies in their ability to treat large areas of skin and target subclinical AKs. This concept of “field treatment” is extremely attractive for a large number of patients who have diffuse actinic damage over large portions of their skin. It becomes difficult to directly compare various treatment modalities; however, because of various endpoints used in many studies. Several recent split-face investigations have attempted to address this issue.
5-Fluorouracil
5-fluorouracil (5-FU) is available in several topical formulations of varying strengths. As an antimetabolite, it inhibits DNA synthesis in dividing cells, preferentially targeting AKs over normal skin cells. Because it is applied topically as field treatment, its advantages include the ability to treat clinical and subclinical lesions over a period of 2 to 4 weeks with minimal risk of scarring. However, as a patient-applied agent, its success is dependent upon patient adherence. Because it is a potent irritant, associated pain, photosensitivity, or discomfort may curtail its proper use. As a stand-alone agent, it has been shown to completely clear close to 50 percent of lesions treated daily for four weeks with moderate-to-severe irritation being reported by 90 percent of users.13 Jorizzo et al demonstrated a significant improvement in clearance of AKs when 5-FU was used sequentially after cryosurgery, with 30 percent of patients treated remaining clear at six months after treatment.14
Imiquimod
Imiquimod, available as a 5% cream, is an immune-response modifier that is US Food and Drug Administration-approved for the treatment of AKs. By inducing the response of toll-like receptors located within the epidermis, it allows the patient’s own innate immune system to become more effective in controlling the growth of AKs. With its use over the past 10 years, several large, multicenter, placebo-controlled, double-blind studies have established its effectiveness as a first-line agent for the treatment of AKs.15–18 Large, Phase 3 studies have demonstrated imiquimod’s efficacy in treating AKs, with an 83-percent median reduction in lesions for the treated arm versus zero percent for the vehicle group. Medication was applied once per day, two days per week, for 16 weeks.15 An additional study utilizing imiquimod three times per week for four weeks followed by four weeks of rest resulted in 50 percent of patients achieving complete clearance of lesions and 75 percent reaching partial clearance after two treatment cycles.19 Zeichner et al demonstrated that application once per week for six months also resulted in high clearance rates for AKs.17 Collectively, these studies indicate that imiquimod is quite effective at reduced application frequencies or in cyclical fashion. Furthermore, given the variability in patient response to imiquimod, the observed inflammatory response as opposed to strict adherence to a treatment protocol may be the best guide when using this agent. In those who are “strong responders,” it may be sensible to discontinue therapy early and restart in an additional 2 to 4 weeks if clinically indicated.
Perhaps the best indication for the use of imiquimod in the treatment of AKs lies in its sustained effect. Lee et al demonstrated sustained efficacy as far as 16 months after initial treatment with imiquimod and suggest that this may be related to modification in immune function and or immune memory.20 Of the 146 patients followed after treatment of AKs with imiquimod, only 25 percent had new or recurrent lesions 16 months after treatment. Krawtchenko et al devised a randomized study of topical imiquimod versus 5-FU versus cyrotherapy and compared both clinical and histological outcomes initially and at one year. Cryotherapy was performed for 20 to 40 seconds followed by a second treatment if the lesion was not fully clear in two weeks. Five percent 5-FU was applied twice a day for four weeks with a rest period of up to one week in the case of acute inflammation. Imiquimod was applied three times per week for four weeks followed by a four-week rest period and was repeated if any lesions were clinically apparent. Patients were randomized to 1 of the 3 treatment groups and clinical clearance was achieved in 68 percent of those treated with cryosurgery, 96 percent with 5-FU, and 85 percent with imiquimod. Histological clearance rate was 32 percent for cryosurgery, 67 percent for 5-FU, and 73 percent for imiquimod. The one-year sustained clearance rate was 28 percent for cryosurgery, 54 percent for 5-FU, and 73 percent for imiquimod. The patients treated with imiquimod were also judged to have superior cosmetic outcomes.18 Because imiquimod relies upon local immune modification and alterations in the cytokine milieu as opposed to a toxic effect on individual keratinocytes, it is unique in that it does not have to reach each cell within a given lesion to be effective. This may contribute to its high treatment success rate and sustained response.
Side effects related to imiquimod use include flu-like symptoms in a subgroup of users, irritation, scaling, and erythema that may persist beyond treatment discontinuation.15,16
Photodynamic Therapy
Photodynamic therapy (PDT) affords yet another unique and effective approach for the treatment of AKs. With its proven success and excellent cosmetic outcome, its use has been increasing in popularity. It relies on a two-step procedure consisting of the application of a photosensitizing agent (either aminolevulinic acid [ALA] or methyl aminolevulinate [MAL]) that is taken up by cells of the skin and converted to a porphyrin molecule [protoporphyrin IX]). An external light source (either blue or red light) is then used to activate the porphyrins resulting in the creation of destructive reactive oxygen species (ROS) and cell death. This process preferentially occurs at greater frequency in malignant and premalignant cells, allowing for targeted clearance of AKs and nonmelanoma skin cancers. A large number of variables exist for the use of PDT (Table 3), and the optimal combination of these parameters has yet to be determined. The American Academy of Dermatology has recently published an evidence-based international consensus for guidelines on the use of PDT for nonmelanoma skin cancers, including AKs.21 The authors review each of the main clinical trials using PDT to treat AKs and conclude that, “PDT with MAL and ALA is highly effective for treatment of AKs, offering excellent cosmetic results, and should be considered as first-line therapy.” In a randomized, placebo-controlled, Phase 3 study, 243 patients received either vehicle or ALA followed by PDT within 14 to 18 hours. Clinical response rate was based on complete clearing of 75 percent of lesions measured at Week 8 and Week 12. Of the PDT-treated group, 77 percent had a complete response by Week 8 and 89 percent by Week 12. This compared to 18 percent and 13 percent for the placebo group.22 In another large, multicenter study, a split-face technique compared the use of MAL-PDT to cryotherapy. PDT resulted in a higher rate of cured lesions (87% versus 76% lesion reduction from baseline) and; furthermore, subjects and investigators preferred PDT and felt it had a better cosmetic outcome. Both treatment regimens were deemed safe and well tolerated.23 During PDT treatment, patients most frequently complain of stinging, burning, and/or pain that subsided by 24 hours. Erythema and edema of treated areas were common, but cleared by four weeks post-treatment.22
Table 3.
Variables in the use of photodynamic therapy (PDT) for actinic keratoses
| Sensitizer: aminolevulinic acid (ALA) versus |
| methyl aminolevulinate (MAL) |
| Duration of application of sensitizer |
| Light source (laser, LEDS, IPL) |
| Light type (red vs. blue light) |
| Light dose |
| Number of treatments |
| Occlusion of sensitizer |
| Single lesion versus field treatment |
Diclofenac
Diclofenac is a topical nonsteroidal anti-inflammatory drug (NSAID) that has been used for the treatment of AKs. Although its precise mechanism of action is unclear, it is believed that beneficial effects are mediated through inhibition of the cyclooxygenase pathway. As topical treatment for AKs, diclofenac exhibits markedly less irritation than agents such as 5-FU and imiquimod, at the concession of lower efficacy rates and longer treatment duration. In a recent meta-analysis of its use in the treatment of AKs, complete response rates were 39 percent, with a mean treatment duration of 75±21 days. Mild-to-moderate skin irritation was the major side effect of the treatment group.24 Berlin et al presented a prospective, double-arm, multicenter, open-label, Phase 4 study aimed to determine the efficacy of sequential therapy of AKs with cryosurgery followed by diclofenac applied daily for 90 days. Forty-six percent of subjects who underwent combination treatment achieved 100-percent lesion clearance compared to 21 percent in the cryosurgery alone arm. This is a good example of newer strategies involving combinations of established treatment modalities.25
When Should an Actinic Keratosis be Biopsied or Excised?
Although typically not warranted, there are situations where the clinician should have a lower threshold to biopsy suspicious lesions or employ more aggressive treatment options. It may be difficult to distinguish AKs from invasive cancer in patients with a history of multiple skin cancers and diffuse actinic damage, particularly those who are immunosuppressed. Rapid growth or thickening of lesions, hyperkeratosis, symptoms such as tenderness or bleeding, and recalcitrance to established treatment modalities are among the factors that should be taken into account when considering more invasive approaches to characterize and treat AKs. Treatment-resistant AKs may be removed by curettage or shave excision. Curettage can be used alone or in conjunction with electrosurgery, cryotherapy, or chemical treatments. Surgical excision is particularly useful for lesions suspected of being SCCs because this technique enables the clinician to treat the lesion appropriately and accurately establish the diagnosis.
When multiple recalcitrant lesions are present, biopsy and/or excision of multiple excisions becomes inappropriate and can result in significant morbidity to the patient. In such situations, field treatment of a more invasive or physical nature may be of benefit. Among these options are laser resurfacing, dermabrasion, and chemical peels. Iyer et al demonstrated a lesion-free period of greater than one year in 21 of 24 patients treated with either CO2 or Er:YAG full-face laser resurfacing.26 Similar degrees of efficacy and sustained results have been shown by others.27,28 In a randomized, prospective trial comparing Er:YAG resurfacing with topical 5-FU, there were significantly less recurrences in the laser group as compared with the 5-FU group. Side effects were more common among those with laser resurfacing and included erythema and hypopigmentation.29 When chemical peels were investigated, medium-depth peels, such as Jessner’s solution plus 35% trichloroacetic acid, were shown to have similar efficacy as topical 5-FU, implying that deeper peel depth is necessary for treatment of stubborn or more advanced lesions.30 Finally, dermabrasion has been demonstrated to provide long-term (>5 year) clearance of AKs in a small, retrospective study.31 The relative paucity of large studies investigating these treatment options makes accurate assessment of their utility difficult and further work in these areas is particularly warranted.
Conclusion
AKs are an indication of UV-induced damage to the skin, as they represent strong predictors for the development of SCC and to a lesser degree, basal cell carcinoma. As such, treatment of AKs may prevent the progression to invasive cutaneous malignancies. Many treatment options exist and attributes of both the patient and provider influence the optimal method for a given clinical situation. Each treatment for AKs has its unique advantages and disadvantages. The variety of highly effective treatment modalities available is a great asset to the dermatologist and practitioners should not be adverse to using multiple approaches alone or in combination to maximize efficiency, long-term efficacy, and patient satisfaction.
References
- 1.Spencer JM, Hazan C, Hsiung SH, Robins P. Therapeutic decision making in the therapy of actinic keratoses. J Drugs Dermatol. 2005;4(3):296–301. [PubMed] [Google Scholar]
- 2.Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med. 993(14):1147–1151. doi: 10.1056/NEJM199310143291602. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155(1):9–22. doi: 10.1111/j.1365-2133.2005.07121.x. [DOI] [PubMed] [Google Scholar]
- 4.Ehrig T, Cockerell C, Piacquadio D, Dromgoole S. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatol Surg. 2006;32(10):1261–1265. doi: 10.1111/j.1524-4725.2006.32287.x. [DOI] [PubMed] [Google Scholar]
- 5.Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 Pt 2):23–24. doi: 10.1067/mjd.2000.103339. [DOI] [PubMed] [Google Scholar]
- 6.Marks R. The role of treatment of actinic keratoses in the prevention of morbidity and mortality due to squamous cell carcinoma. Arch Dermatol. 1991;127(7):1031–1033. [PubMed] [Google Scholar]
- 7.Hurwitz RM, Monger LE. Solar keratosis: an evolving squamous cell carcinoma. Benign or malignant? Dermatol Surg. 1995;21(2) doi: 10.1111/j.1524-4725.1995.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 8.Marks R, Foley P, Goodman G, et al. Spontaneous remission of solar keratoses: the case for conservative management. Br J Dermatol. 1986;155:649–655. doi: 10.1111/j.1365-2133.1986.tb06644.x. [DOI] [PubMed] [Google Scholar]
- 9.Dodson JM, DeSpain J, Hewett JE, Clark DP. Malignant potential of actinic keratoses and the controversy over treatment. A patient-oriented perspective. Arch Dermatol. 1991;127(7):1029–1031. [PubMed] [Google Scholar]
- 10.Warino L, Tusa M, Camacho F, et al. Frequency and cost of actinic keratosis treatment. Dermatol Surg. 2006;32(8):1045–1049. doi: 10.1111/j.1524-4725.2006.32228.x. [DOI] [PubMed] [Google Scholar]
- 11.Darlington S, Williams G, Neale R, Frost C, Green A. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol. 2003;139(4):451–455. doi: 10.1001/archderm.139.4.451. [DOI] [PubMed] [Google Scholar]
- 12.Thai K, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687–692. doi: 10.1111/j.1365-4632.2004.02056.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2 or 4 weeks. Cutis. 2002;70:22–29. [PubMed] [Google Scholar]
- 14.Jorizzo J, Weiss J, Furst K, VandePol C, Levy SF. Effect of a 1 week treatment with 0.5% topical fluorouracil on occurrence of actinic keratosis after cryosurgery. Arch Dermatol. 2004;140(7):813–816. doi: 10.1001/archderm.140.7.813. [DOI] [PubMed] [Google Scholar]
- 15.Lebwohl M, Dinehart S, Whiting D, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. Arch Dermatol. 2004;140:813–816. doi: 10.1016/j.jaad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251–1255. doi: 10.1038/sj.jid.5700264. [DOI] [PubMed] [Google Scholar]
- 17.Zeichner JA, Stern DW, Uliasz A, Itenberg S, Lebwohl M. Placebo-controlled, double-blind, randomized pilot study of imiquimod 5% cream applied once per week for 6 months for the treatment of actinic keratoses. J Am Acad Dermatol. 2009;60(1):59–62. doi: 10.1016/j.jaad.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Krawtchenko N, Roewert-Huber J, Ulrich M. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clincal and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(2):34–40. doi: 10.1111/j.1365-2133.2007.08271.x. [DOI] [PubMed] [Google Scholar]
- 19.Rivers JK, Rosoph L, Provost N, Bissonnette R. Open-label study to assess the safety and efficacy of imiquimod 5% cream applied once daily three times per week in cycles for treatment of actinic keratoses on the head. J Cutan Med Surg. 2008;12(3):97–101. doi: 10.2310/7750.2008.07045. [DOI] [PubMed] [Google Scholar]
- 20.Lee PK, Harwell WB, Loven KH. Long-term clinical outcomes following treatment of actinic keratosis with imiquimod 5% cream. Dermatol Surg. 2005;31(6):659–664. doi: 10.1111/j.1524-4725.2005.31608. [DOI] [PubMed] [Google Scholar]
- 21.Braathen LR, Szeimies R, Basset-Seguin N, et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. J Am Acad Dermatol. 2007;56(1):125–143. doi: 10.1016/j.jaad.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Piacquadio DJ, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140(1):41–46. doi: 10.1001/archderm.140.1.41. [DOI] [PubMed] [Google Scholar]
- 23.Morton C, Campbell S, Gupta G, et al. AK. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol. 2006;155(5):1029–1036. doi: 10.1111/j.1365-2133.2006.07470.x. [DOI] [PubMed] [Google Scholar]
- 24.Pirard D, Vereecken P, Melot C, Heenen M. Three percent diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses: a meta-analysis of the recent studies. Arch Dermatol Res. 2005;297(5):185–189. doi: 10.1007/s00403-005-0601-9. [DOI] [PubMed] [Google Scholar]
- 25.Berlin JM, Rigel DS. J Diclofenac sodium 3% gel in the treatment of actinic keratoses postcryosurgery. J Drugs Dermatol. 2008;7(7):669–673. [PubMed] [Google Scholar]
- 26.Iyer S, Friedli A, Bowes L, Kricorian G, Fitzpatrick RE. Full face laser resurfacing: therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:114–119. doi: 10.1002/lsm.20012. [DOI] [PubMed] [Google Scholar]
- 27.Sherry SD, Miles BA, Finn RA. Long-term efficacy of carbon dioxide laser resurfacing for facial actinic keratosis. J Oral Maxillofac Surg. 2007;65(6):1135–1139. doi: 10.1016/j.joms.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Ostertag JU, Quaedvlieg PJ, Neumann MH, Krekels GA. Recurrence rates and long-term follow-up after laser resurfacing as a treatment for widespread actinic keratoses on the face and scalp. Dermatol Surg. 2006;32(2):261–267. doi: 10.1111/j.1524-4725.2006.32046.x. [DOI] [PubMed] [Google Scholar]
- 29.Ostertag JU, Quaedvlieg PJ, van der Geer S, et al. A clinical comparison and long-term follow-up of topical 5-fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers Surg Med. 2006;38(8):731–739. doi: 10.1002/lsm.20379. [DOI] [PubMed] [Google Scholar]
- 30.Witheiler DD, Lawrence N, Cox SE, et al. Long-term efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Dermatol Surg. 1997;23:191–196. doi: 10.1111/j.1524-4725.1997.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 31.Coleman WP, Yardborough JM, Mandy SH. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22:17–21. doi: 10.1111/j.1524-4725.1996.tb00565.x. [DOI] [PubMed] [Google Scholar]


