Table 1.
Effect of Some Reaction Parameters on the Asymmetric Suzuki Arylation of a Racemic α-Chloroamide.
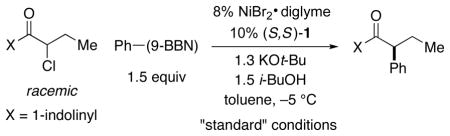 | |||
|---|---|---|---|
| entry | variation from the “standard” conditions | ee (%) | yield (%)a |
| 1 | none | 92 | 89 |
| 2 | no NiBr2·glyme | – | <2 |
| 3 | no (S,S)-1 | – | 8 |
| 4 | no i-BuOH | – | 8 |
| 5 | water, instead of i-BuOH | 91 | 25 |
| 6 | (S,S)-2, instead of (S,S)-1 | 85 | 74 |
| 7 | r.t. | 82 | 84 |
| 8 | 4% NiBr2· glyme, 5% (S,S)-1 | 91 | 78 |
| 9 | X = NBnPh | – | 6 |
| 10 | X = NPh2 | – | 4 |
| 11 | X = NEt2 | 76 | 19 |
| 12 | X = NMe(OMe) | <5 | 84 |
| 13 | X = NHMe | 26 | 82 |
| 14 | X = OEt | 50 | 74 |
All data are the average of two experiments.
The yield was determined by GC or 1H NMR analysis versus an internal standard.
