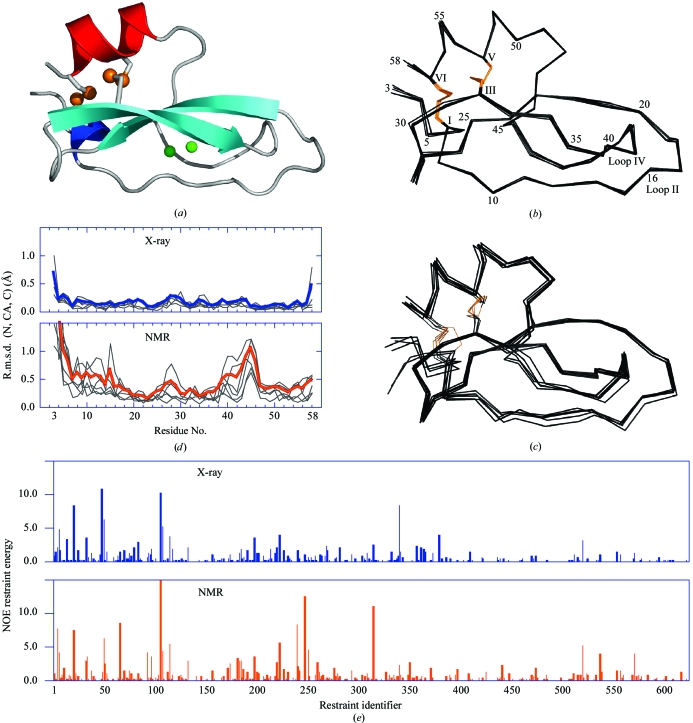
Figure 3.
Conkunitzin-S1 crystal structure overview and comparison with NMR-determined models. (a) Conkunitzin-S1 folds into the 310–β–β–α fold characteristic of Kunitz domains. S atoms in each of two disulfide bonds are shown as orange spheres. Two water molecules, shown as green spheres, are completely buried inside conkunitzin-S1. The secondary-structure elements are colored with the 310-helix blue, β-strands cyan and α-helix red. (b) Cα superposition of the six conkunitzin-S1 molecules found in the asymmetric unit. (c) Cα superposition of the six unique NMR-ensemble members currently available (PDB code 1yl2). In (b) and (c) disulfide bonds are shown with orange lines, selected residues are labeled with Arabic numerals and cysteines are labeled with Roman numerals. (d) R.m.s. deviations of main-chain atoms (N, CA and C) are plotted as a function of residue number for crystal (top panel) and NMR models (bottom panel). (e) Energies computed for individual NOE distance restraints are plotted as a bar graph, with blue and orange bars indicating the energies computed for representative X-ray-determined and NMR-determined structures. (a), (b) and (c) were generated using PyMOL (DeLano, 2002 ▶).