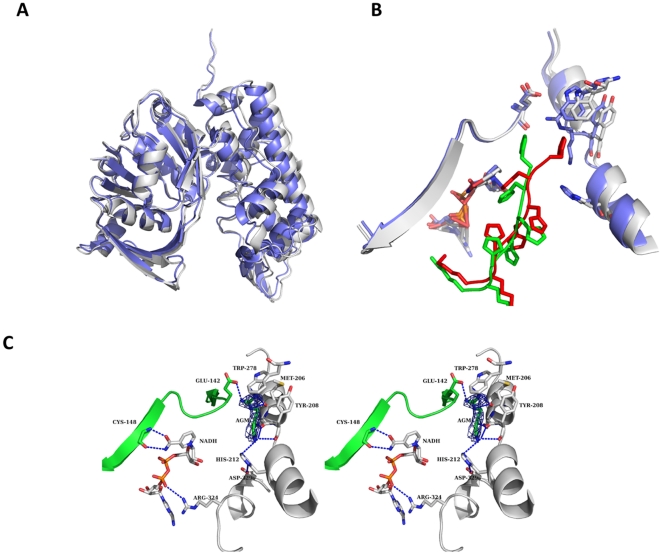
Figure 5. Structural comparison A) Superposition of the OcDH/NADH (white) and the OcDH/NADH-agmatine (blue) complexes highlight the inward rotation of domain II.
B) Superposition of the agmatine binding site. Shown is the binding site in the OcDH/NADH complex (white) and from the OcDH/NADH-agmatine complex (blue).the reorientation of the his5-tag is shown by the representation of both his5-tag (red for the OcDH/NADH complex and green for OcDH/NADH/-agmatine complex) C) Stereoview of the agmatine-binding pocket of OcDH. The agmatine-binding pocket is located in domain II, directly at the N-terminal helix-kink-helix motif of domain II. Agmatine is coordinated by the side chain Trp278, backbone interactions with Tyr208 (domain II) and Glu142 (domain I). NADH is bound in a canonical fashion to the Rossman fold, while interactions of the amide side chain of the nicotine amide ring with the backbone atoms of Cys148 (domain I) ensure the syn-conformation. The electron density of an omit map contoured at 1 σ for agmatine is shown as blue mesh.