Table I.
Observed compounds resulting from the reaction between diphenylphophine selenide and metal carboxylates.
| δ (ppm)a |
δ (ppm)a |
||
|---|---|---|---|
| 2 RCOOH | 12.2 - 12.5b,e | 7 Ph2PH | - 40.2c,e 215 Hz 1J(31P - 1H) |
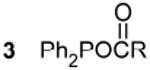 |
98.9c,g | 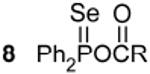 |
77.1c,h 875 Hz 1J(31P - 77Se) |
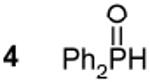 |
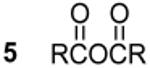
22.0c,e 441 Hz 1J(31P - 1H) | 12 Ph2PPPh2 | - 14.5c,e,f |
 |
168.9d,e | 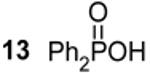 |
25.7c,e,f |
| R=C17H33 | |||
All spectra were recorded in toluene-d8
1H NMR
31P NMR
13C NMR.
Verified by addition of authentic sample.
Isolated diffraction quality single crystals from the reaction.
Chemiker Zeitung 1982, 391-395.
Chem. Commun., 2005, 2692-2694.
