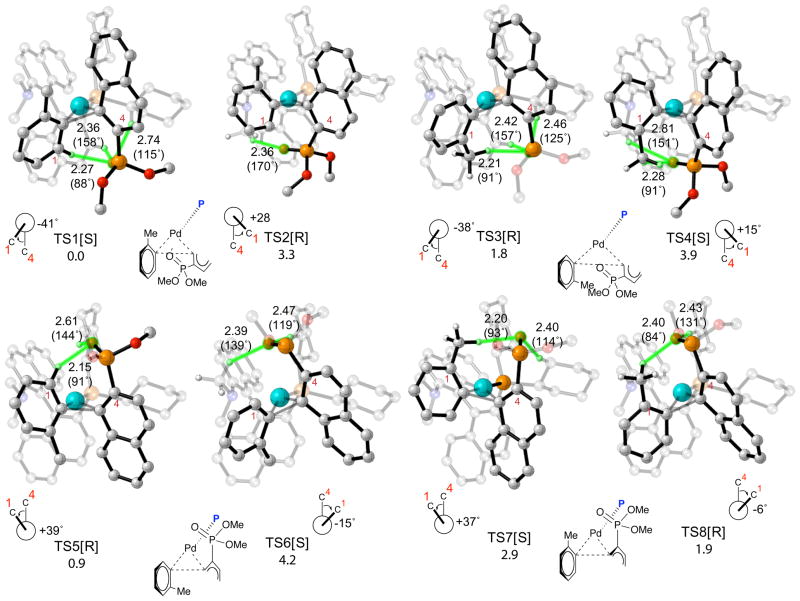
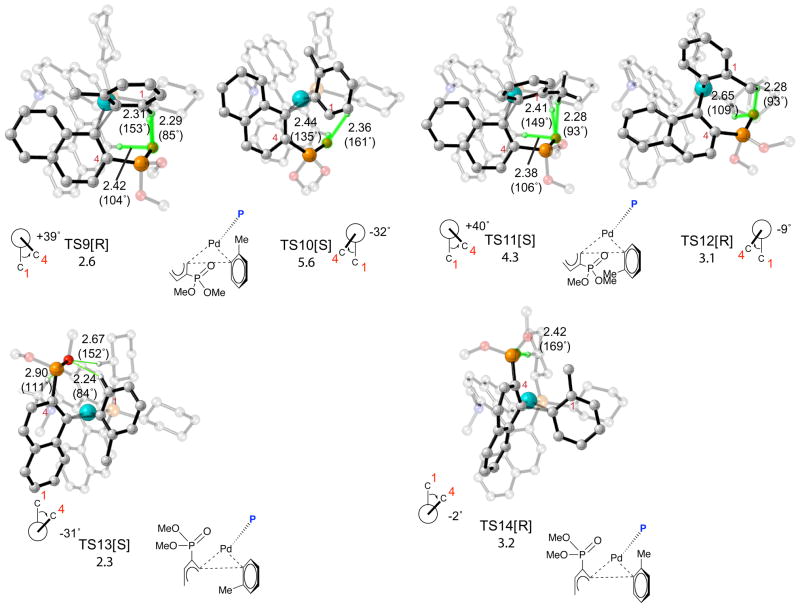
Figure 6.
Transition structures and relative free energies of activation in kcal/mol for reductive elimination of biaryl phosphines from Pd(KenPhos) complexes formed after transmetalation. Atoms participating in H-bonding interactions are highlighted in green. The stereochemistry of the product formed after reductive elimination is denoted in brackets. Relevant O-H bond distances in Å are shown on the pictures; H-O-P bond angles in degrees are shown in parentheses. Chemdraw representations of the transition structures are shown below the pictures; the KenPhos ligand in these drawings has been replaced by the symbol P and the naphthylphosphonate addend has been truncated for clarity. Transition structures and relative free energies of activation in kcal/mol for reductive elimination of biaryl phosphines from Pd(KenPhos) complexes formed after transmetalation. Atoms participating in H-bonding interactions are highlighted in green. The stereochemistry of the product formed after reductive elimination is denoted in brackets. Relevant O-H bond distances in Å are shown on the pictures; H-O-P bond angles in degrees are shown in parentheses. Chemdraw representations of the transition structures are shown below the pictures; the KenPhos ligand in these drawings has been replaced by the symbol P and the naphthylphosphonate addend has been truncated for clarity.