Table 1.
Effect of functional group located ortho to the halide substituent on the enantioselectivity.
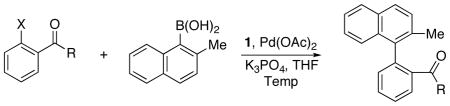 | ||||
|---|---|---|---|---|
| R = NEt2, X = Br (6) | R = N(Me)tBu, X = Br (10) | |||
| R = NEt2, X = I (7) | R = N(OMe)Me, X = Br (11) | |||
| R = NEt2, X = OTf (8) | R = N(H)tBu, X = Br (12) | |||
| R = NiPr2, X = Br (9) | R = N(H)C(Me)2Ph, X = Br (13) | |||
| Entry | ArX | Temp (°C) | Yield %b | ee %c |
| a | 6 | rt | 80 | 75 |
| b | 7 | rt | 78 | 73 |
| c | 8 | rt | 85 | 71 |
| d | 9 | rt | 76 | 82 |
| e | 10 | rt | 82 | 75 |
| f | 11 | rt | 75 | 80 |
| g | 12 | 50 | 68 | 87 |
| h | 13 | 50 | 81 | 93 |
Reaction conditions: 1.0 equiv of aryl halide, 2.0 equiv of boronic acid, 5 mol% Pd, 6 mol% (S)-1, 3 equiv of K3PO4, THF (2.5–3 mL/mmol of halide), 24–40 h.
Isolated yield.
The ee values were determined by chiral HPLC on a Chiralcel OD-H or AD-H.
