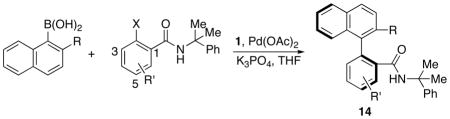
Table 2.
Asymmetric synthesis of biaryls by enantioselective Suzuki-Miyaura coupling.a
 | |||||
|---|---|---|---|---|---|
| Entry | R | R′ | X | Yield %b | ee %c |
| a | Me | H | Cl | 87 | 93(R) |
| b | Me | H | Br | 83 | 93(R) |
| c | Me | H | I | 81 | 94(R) |
| d | OEt | H | Cl | 81 | 91(S) |
| e | OEt | H | Br | 80 | 91(S) |
| f | Me | 4-F | Br | 83 | 93(R) |
| g | OEt | 4-F | Br | 81 | 90(S) |
| h | Me | 4-Me | Br | 82 | 94(R) |
| i | OEt | 4-Me | Br | 81 | 92(S) |
| j | Me | 4-NO2 | Br | 92 | 88(R) |
| k | Me | 5-OMe | Br | 83 | 94(R) |
| l | OEt | 5-OMe | Br | 84 | 91(S) |
| m | Me | 5-CF3 | Br | 87 | 89(R) |
| n | Me | 5-F | Br | 85 | 92(R) |
| o | Me | 6-Me | Br | 80 | 48(R) |
| p | Me | 6-F | Br | 46 | 90(R) |
Reaction conditions: 1.0 equiv of aryl halide, 1.5–2.0 equiv of boronic acid, (S)-1/Pd(OAc)2 = 1.2, 3 equiv of K3PO4, THF (2.5–3 mL/mmol of halide).
Isolated yield (average of two runs).
The ee values were determined by chiral HPLC on a Chiralcel OD-H or AD-H.
The absolute configuration of 14h was determined by X-ray crystallography. The configurations of other biaryl compounds were assigned by analogy.
