Fig. 1.
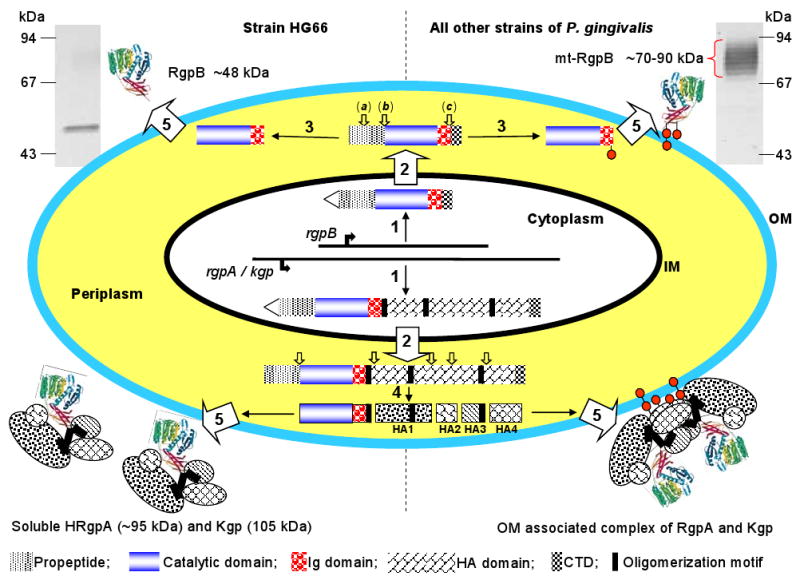
Schematic diagram of discrete steps in gingipain secretion, processing, posttranslational maturation, and assembly of extracellular gingipain complexes. Western blot analysis (upper corners) of the whole culture of P. gingivalis strain HG66 and W83 using anti-RgpB mAb illustrates the difference in the molecular mass between soluble and membrane-attached RgpB. [1] Gingipain genes are translated into a polypeptide chain composed of the classical signal peptide (triangle), a propeptide, a catalytic domain, an Ig-like domain, a hemagglutinin-adhesin domain (missing in RgpB) and a C-terminal domain. Black bars in Kgp/RgpA denote oligomerization motifs involved in non-covalent association of individual domains of the HRgpA/Kgp complex (200). [2] Nascent translation products are translocated across the inner membrane (IM) by the Sec system with simultaneous cleavage of the signal peptide. [3] In the periplasm, pro-RgpB undergoes three steps of sequential processing (from a to c, open arrows). First N-terminal propeptide is cleaved off in two steps (a and b) then the C-terminal domain is removed (c). Each processing step requires the previous step leading to incremental enhancement of the activity in a stepwise manner (123). The intact C-terminal domain, prior to its processing, is essential for RgpB maturation and secretion (136, 189). In strain HG66, non-glycosylated RgpB is released into extracellular milieu in the soluble form; in all other strains, RgpB is glycosylated and remain bound to the cell-surface. Small circles indicate lipopolysaccharide-like glycan moieties for membrane anchorage. [4] RgpA and Kgp processing is more complicated than that of RgpB. In addition to the propeptide removal, several cleavages within hemagglutinin-adhesin (HA) (open arrows) liberate individual functional domains which remain non-covalently associated. Concurrent with the proteolytic processing or the outer membrane (OM) translocation, a glycan moiety is attached to the HA4 domain for membrane anchorage. [5] Gingipains traverse the outer membrane using a unique secretion pathway apparently via a novel outer membrane translocase apparatus (185).
