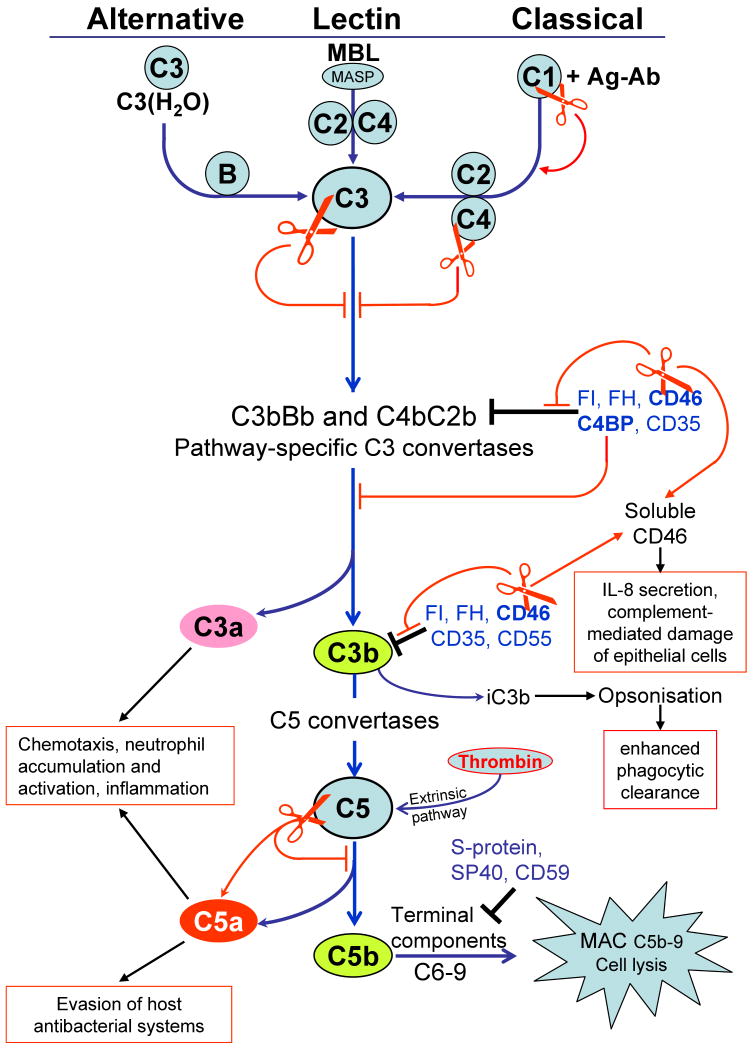
Fig. 2.
Activation pathways of the complement system and points of gingipain intervention. Complement is initiated by three major pathways. The alternative pathway is triggered due to a failure to appropriately regulate constant low-level spontaneous deposition of activated C3 on foreign surfaces. Spontaneously hydrolyzed C3 forms a complex with factor B (fB) leading to formation of the initial alternative pathway C3 convertase (C3bBb). The lectin pathway is initiated by the binding of mannan binding lectin (MBL) to mannose residues on the surface of microorganisms which activates mannan-binding lectin serine proteases (MASP), which then cleave C4 and C2. The classical pathway is activated when antibodies bind to their corresponding antigen and serine proteases (C1s and C1r) in C1 complex are activated which then cleave C4 and C2. As a result, in both pathways the same C3 convertase (C4bC2b) is formed. All three pathways converge at a central step, involving activation of the third component of complement (C3) leading to the generation of the anaphylatoxin C3a and opsonins C3b and iC3b. In the terminal pathway, C5b initiates the assembly of the C5b–9 membrane attack complex (MAC), which in turn induces microbial cell lysis. Host surfaces are protected from spontaneous complement activation by complement regulators acting at several stages of complement activation pathway (black end lines). The complement regulators are either cell-surface associated proteins, such as CD35 (Complement Receptor 1, CR1), CD46 (membrane cofactor protein, MCP), CD55 (decay accelerating factor, DAF) and CD59 or soluble regulators circulating in blood, including factor H (FH), factor I (FI), C4 binding protein (C4BP), S-protein (vitronectin), and SP-40 (clusterin) (245). Targets for gingipain attack are depicted by red scissors resulting either in the inhibition of the pathway (red end lines) or stimulation of the pathway (thin red arrows).