Figure 1.
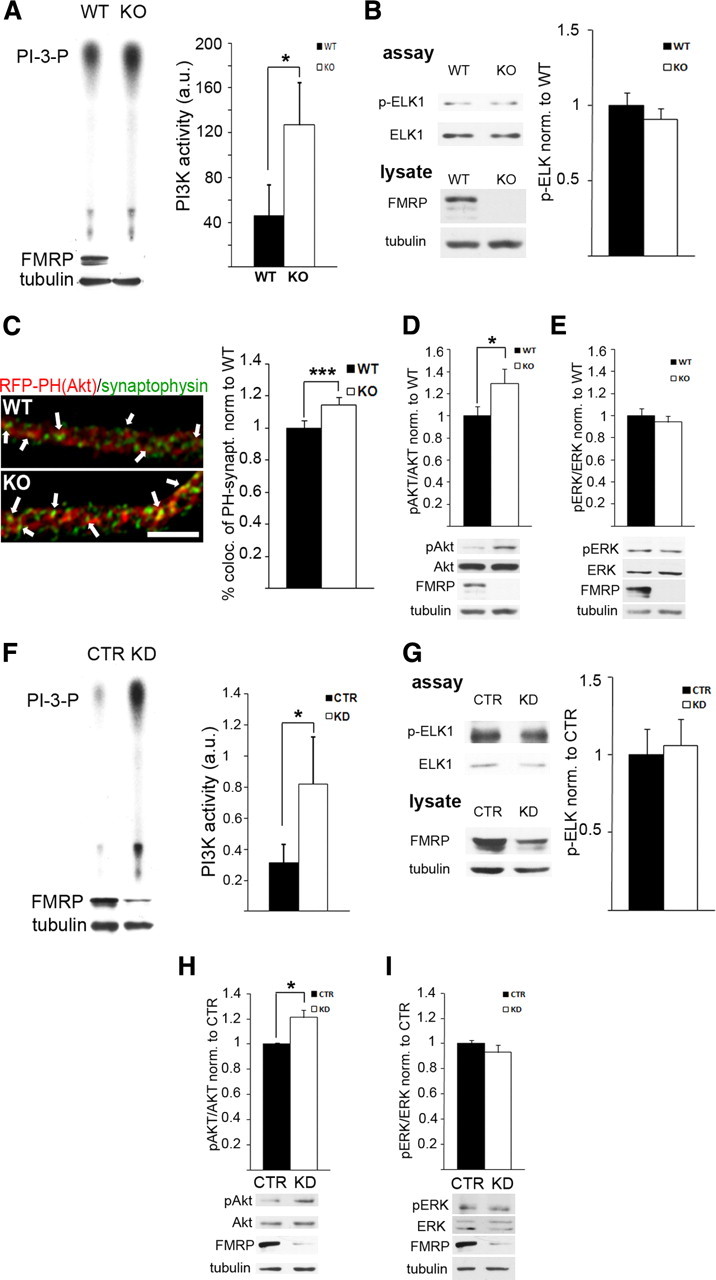
A–E, Exaggerated PI3K activity and signaling at Fmr1 KO synapses. A, PI3K activity is threefold increased in Fmr1 KO SNS compared with WT SNS, as assessed by the amount of radiolabeled PI3-P on autoradiographies from in vitro PI3K assays (n = 8, *p = 0.035, paired t test) (control experiment shown in supplemental Fig. S1A, available at www.jneurosci.org as supplemental material). B, Quantification of phospho-ELK-1 specific Western blots of in vitro ERK1/2 kinase assays from WT and Fmr1 KO SNS using recombinant ELK-1 as a substrate shows no significant change in ERK1/2 activity in the absence of FMRP (n = 8, p = 0.20, paired t test) (control experiment shown in supplemental Fig. S1B, available at www.jneurosci.org as supplemental material). FMRP-specific Western blot analyses of the lysate used as starting material for the assay are shown below; tubulin served as loading control. C, Quantitative analysis of recombinant RFP–PH(Akt) domain colocalized with synaptophysin in hippocampal neurons (9–10 DIV) demonstrates increased synaptic localization of RFP–PH(Akt) in Fmr1 KO neurons, suggesting elevated PI3-P3 levels at Fmr1 KO synapses, which lead to a translocation of the fluorescently labeled PH domain (scale bar, 5 μm) (n = 5 independent experiments, 6–12 cells each, ***p = 0.00002, paired t test). In contrast, total dendritic RFP–PH(Akt) levels were not different between WT and Fmr1 KO neurons (supplemental Fig. S1C, available at www.jneurosci.org as supplemental material). D, E, Phosphorylation of the PI3K downstream signaling molecule Akt (D), but not ERK1/2 (E), is significantly increased in Fmr1 KO SNS compared with WT. Phosphorylation levels were assessed by densitometric analysis of Western blots, and phospho-specific signals were normalized to total levels of the respective proteins (D, n = 6, *p = 0.014; E, n = 7, p = 0.18; paired t tests). Representative Western blots are shown below. F–I, PI3K activity and signaling is increased in FMRP-deficient HEK293T cells. In contrast to cortical SNS, HEK293T cells do not express detectable levels of mGluR1 and mGluR5 receptors (Western blot analyses shown in supplemental Fig. S1D, available at www.jneurosci.org as supplemental material). F, G, siRNA-mediated knockdown of Fmr1 in HEK293T cells increases PI3K activity significantly (F, n = 7, *p = 0.039, paired t test), whereas in vitro ERK1/2 assays showed no significant change in activity (G, n = 7, p = 0.32, paired t test). H, I, Quantification of Akt and ERK1/2 phosphorylation after Fmr1 knockdown demonstrate significantly enhanced phospho-Akt levels (H, n = 5, *p = 0.018, paired t test) but not phospho-ERK1/2 levels (I, n = 6, p = 0.29, paired t test). Genotype or siRNA-mediated knockdown was confirmed by Western blotting with an FMRP-specific antibody and a tubulin antibody as loading control (shown below for each experiment). All error bars represent SEM. a.u., Arbitrary unit.
