Figure 8.
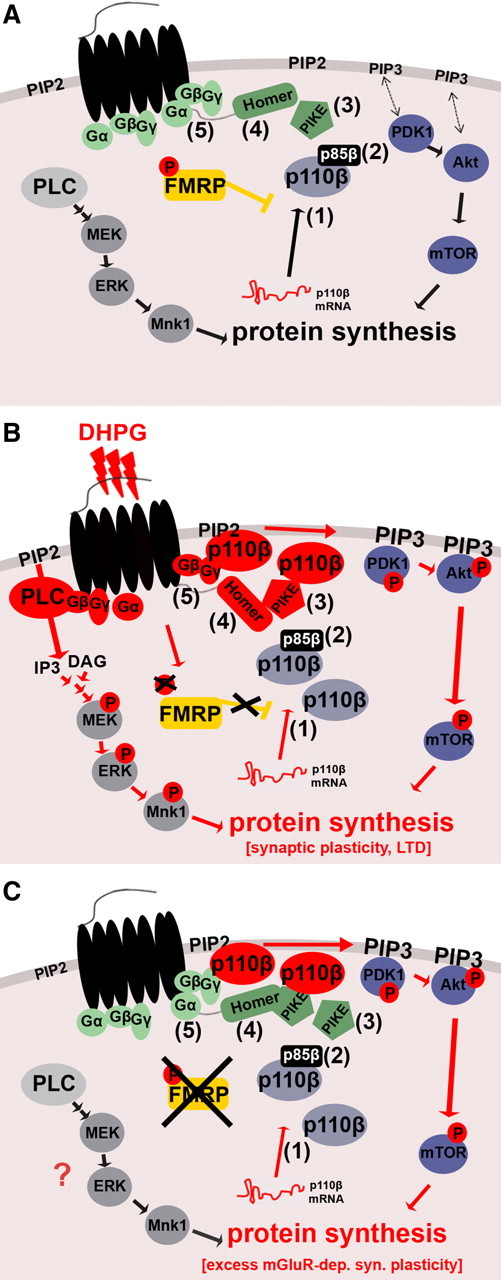
Proposed model for dysregulated mGluR signaling in FXS. A, B Regulation of the gp1 mGluR-dependent signal pathways PLC/ERK and PI3K/mTOR in WT. A, Under basal conditions, FMRP puts the break on PI3K activity. This is partially attributable to FMRP-mediated repression of p110β mRNA translation and synaptic localization of the catalytic subunit p110β (1). Additional mechanisms might include regulation of p110β-modulating subunits such as p85β (2) and PIKE (3) by FMRP. B, gp1 mGluR-mediated activation of PLC and PI3K pathways at the synapse. PLC and PI3K share the same substrate PIP2 in the membrane to produce either IP3 and DAG, or PIP3, respectively. The PLC product DAG can activate PKC, leading to induction of the mitogen-activate protein kinase kinase (MEK)/ERK pathway, whereas the PI3K product PIP3 recruits PH-containing kinases PDK1 and Akt to the membrane, thereby inducing their phosphorylation followed by activation of downstream signaling molecules including mTOR. Both pathways induce protein synthesis. During gp1 mGluR stimulation, PLC is activated by small G-proteins. PI3K was shown to be activated by at least two different mechanisms, the Homer–PIKE complex (4) and small G-proteins (5). Furthermore, gp1 mGluR stimulation leads to transient removal of FMRP-mediated translational inhibition by dephosphorylation of FMRP (Ceman et al., 2003; Narayanan et al., 2007, 2008). We hypothesize that, during this time window, synapses experience a twofold “boost” of PI3K activity composed of newly synthesized catalytic p110β subunits (1) as well as activation of preexisting and newly synthesized PI3K subunit molecules via PIKE and G-proteins (4 and 5). Together, enhanced ERK and PI3K activity lead to increased synaptic protein synthesis. C, The “molecular brake” FMRP is absent in Fmr1 KO, and FMRP-mediated inhibition of p110β translation and PI3K activity is removed constitutively. Increased p110β protein levels at synapses (1), which can be activated by basal levels of mGluR signaling (4 and 5), contribute to exaggerated PI3K signaling. Additionally, PI3K activity could be increased by dysregulation of p110β-modulating subunits (2 and 3), especially PIKE, in the absence of FMRP. DHPG-mediated transient increase in p110β protein expression is abolished and may partially account for loss of gp1 mGluR-dependent activation of PI3K signaling. Loss of this combined “brake” on PI3K signaling in FXS would elevate PI3K-dependent protein synthesis to a saturated level, which cannot be further increased by gp1 mGluR stimulation.
