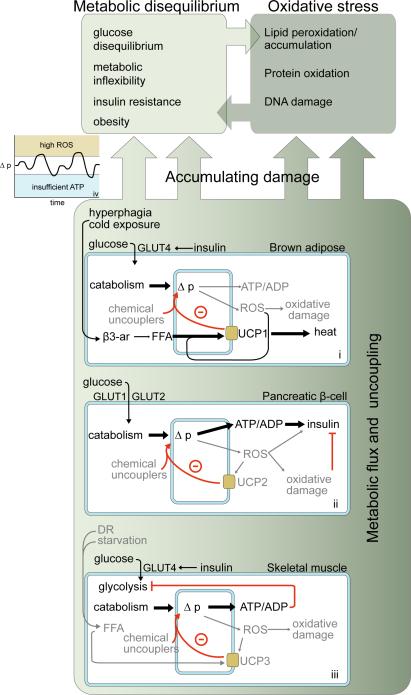
Figure 1.
Examples of different metabolic systems in which biological uncoupling may function. Outer and inner blue shapes represent plasma and mitochondrial membranes, respectively. Black arrows and text denote the primary metabolic mechanisms in each system. (i): In brown adipose tissue, activation of UCP1 by free fatty acids (FFA) dissipates Δp as heat. Loss of UCP1 leads to cold sensitivity and (at thermoneutrality) obesity. (ii): In the pancreatic β-cell, Δp and ATP/ADP fluctuate in response to glucose, allowing glucose-stimulated insulin secretion (GSIS). Uncoupling by UCP2 attenuates GSIS and lowers ROS. UCP2 depletion may therefore lead to short-term increases in GSIS sensitivity, with long-term dysfunction due to oxidative damage. (iii) In skeletal muscle, glucose catabolism is tightly regulated to maintain constant ATP/ADP. Uncoupling by UCP3 may decrease ROS production when ATP demand and therefore Δp is high (e.g., during exercise). (iv): In all cases, a general hypothesis for uncoupling is that it may blunt Δp fluctuation to minimize ROS production. Dysfunction may accumulate faster if the Δp strays from an “optimal” range, while its maintenance within this range may slow cellular damage over time. Whether uncoupling has an ultimately positive or negative impact on lifespan therefore depends on the bioenergetic context in which uncoupling occurs, whether uncoupling acts to maintain or further destabilize metabolic equilibrium, and the control that metabolic equilibrium has over lifespan in an organism.