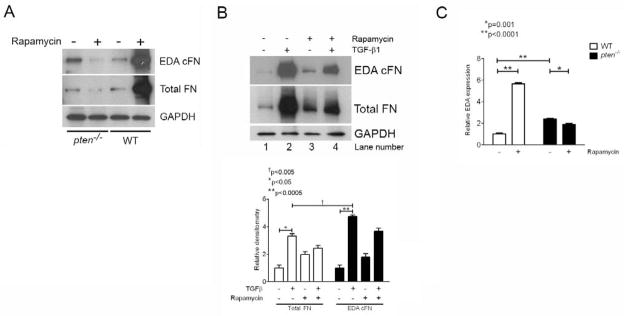
Figure 5. Rapamycin inhibits FN mRNA, FN protein, and endogenous EDA-containing mRNA in pten-deficient cells, but not in WT cells.
(A) Pten−/− or WT cells were treated with or without rapamycin for 24 hours prior to lysis and analysis by Western blot. In pten−/− cells, rapamycin decreased both total and EDA cFN protein production; in contrast, rapamycin enhanced total and EDA cFN protein production in WT cells. MW (KDa) refers to the molecular weight of the identified protein in KDa. The blot is representative of 3 separate experiments. (B) Human fetal lung fibroblasts demonstrate enhanced FN and EDA cFN protein expression with rapamycin treatment (lane 3) compared to untreated cells (lane 1). TGF-β1-stimulated fibroblasts, however, demonstrate decreased FN and EDA cFN protein in the presence of rapamycin (lane 4) compared to cells stimulated with TGF-β1 alone (lane 2). MW (KDa) refers to the molecular weight of the identified protein in KDa. The blot is representative of 3 separately-performed experiments. (C) Rapamycin enhances EDA exon splicing and incorporation into full-length FN in WT cells, but not pten−/− cells. EDA-containing mRNA was normalized to total FN expression to determine the relative effect of rapamycin on EDA splicing. WT (open bars) and pten−/− (closed bars) cells were stimulated with or without rapamycin for 24 hours, and RNA harvested for real-time PCR evaluation of endogenous EDA. Whereas rapamycin treatment increased EDA-containing mRNA significantly in WT cells, it decreased EDA-containing mRNA in pten−/− cells. Note that levels of EDA-containing mRNA in pten−/− cells is approximately 2-fold higher than in WT cells, consistent with our previous findings. Data are pooled from three independent RNA samples performed in triplicate wells. *p=0.001, **p<0.0001.