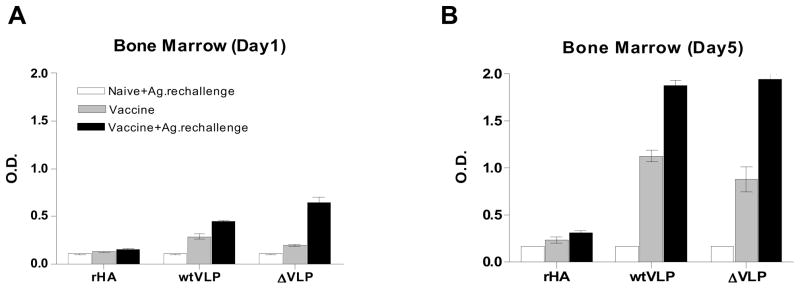
Figure 8. In vitro antibody production during bone marrow cell culture.
Bone marrow cells isolated from vaccinated mice with or without in vivo vaccine Ag. re-challenge were placed on a 96-well plate in quadruplicate and incubated for 5 days without in vitro stimulation. Culture supernatants were harvested on day 1 (A) and day 5 (B) post culture, and influenza specific IgG levels in culture supernatants (2 x diluted) were determined by ELISA. Groups of mice (n=3) were intramuscularly vaccinated with recombinant H5 HA (rHA, 2 μg HA), influenza VLPs containing WT H5 HA (wtVLP, 2 μg HA), or influenza VLPs containing mutant H5 HA (ΔVLP, 2 μg HA) 6 months ago. Control (no pre vaccination), groups of naïve mice (n=3) intramuscularly injected with the corresponding vaccine indicated (2 μg of rHA, wtVLP or ΔVLP containing 0.4 μg HA) 6 days earlier prior to sacrifice. Vaccine: Vaccinated mice (n=3) without in vivo antigenic challenge. Vaccine+Ag.rechallenge, groups of vaccinated mice (n=3) intramuscularly 6 months earlier were re-immunized with the same vaccine (2 μg of rHA, WTVLP or delVLP containing 0.4 μg HA) 6 days prior to sacrifice.