Figure 1. Synthesis and characterization of UAN.
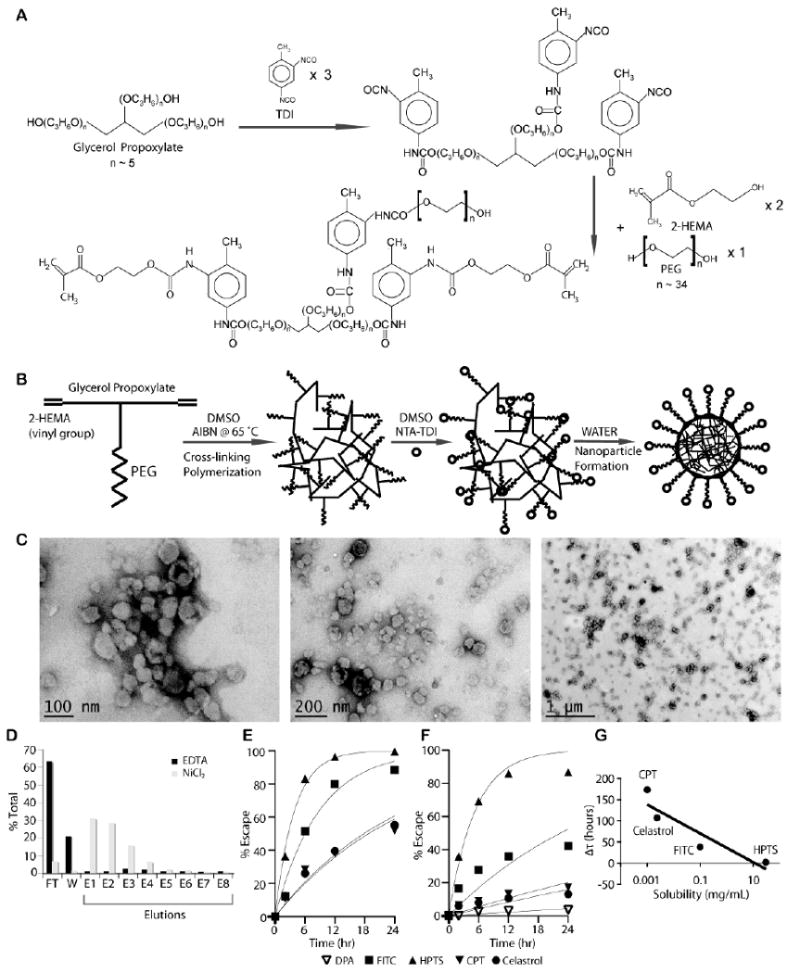
(A) UAN monomer is synthesized by covalently linking 2-HEMA, PEG, and glycerol propoxylate at 2:1:1 molar ratio. (B) NTA-UAN is formed by cross-linking UAN monomers with AIBN and conjugation of NTA to PEG. NTA-UAN forms nanoparticles when suspended in aqueous solution. (C) TEM images of UAN nanoparticles after staining with uranyl acetate. Scale bar is shown. (D) The function and specificity of Ni-NTA on UAN nanoparticles were confirmed by their binding to a His-peptide column. (E-F) The release kinetics of hydrophobic dyes and drugs from UAN were inferred by measuring the rate of escape through dialysis tubes without (E) or with (F) UAN. The fit of the first-order kinetics to the data is shown as a line. (G) The delay in the rate of escape (τ_UAN-τ) of the payloads (HPTS, FITC, celastrol, CPT) is plotted against their respective solubility values in water on a semi-log plot.
