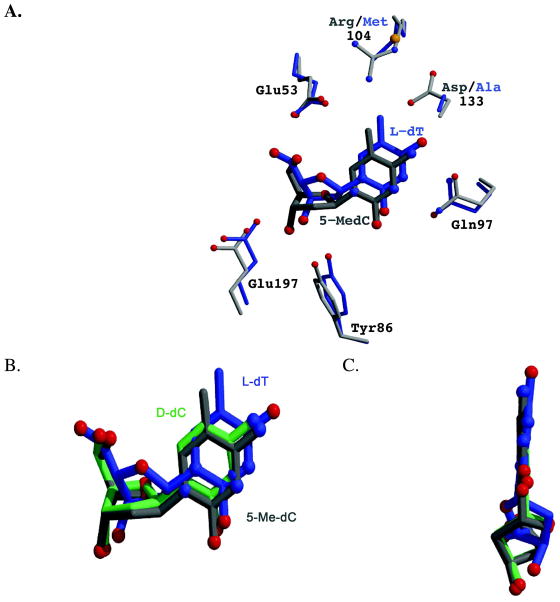
Figure 3.
Comparison of the binding of the nucleosides L-dT (blue), 5-Me-dC (gray) and D-dC (green) to dCK. (A) The L-dT complex was solved with the R104M/D133A variant of dCK. The substitution of Arg104 by a methionine and of Asp133 by alanine increases the active site cavity. Exploiting the increased cavity, L-dT binds deeper into the active site. This repositioning of the nucleoside increases the distance between the 5-methyl group of L-dT and the side-chain of Glu53, thereby eliminating an unfavorable interaction. An analogous movement deeper into the active site is not possible for 5-Me-dC in the context of WT dCK. (B) Overlay of the nucleosides as observed in the structure reported here of WT dCK with 5-Me-dC+ADP (gray), WT dCK with D-dC+ADP (green; PDB ID 1P5Z), and R104M/D133A dCK with L-dT+ADP (blue; PDB ID 3HP1). L-dT binds deeper in the active site due to the larger cavity generated by the R104M/D133A mutations. 5-Me-dC binds slightly lower in this perspective in order to minimize the negative interaction between the 5-methyl group and the side-chains of Glu53 and Arg104. (C) View parallel to the face of the base. The base in L-dT is tilted relative to the base as seen in D-dC and 5-Me-dC. Such base tilting is observed whenever dCK binds nucleosides of the L-chirality.