Figure 9.
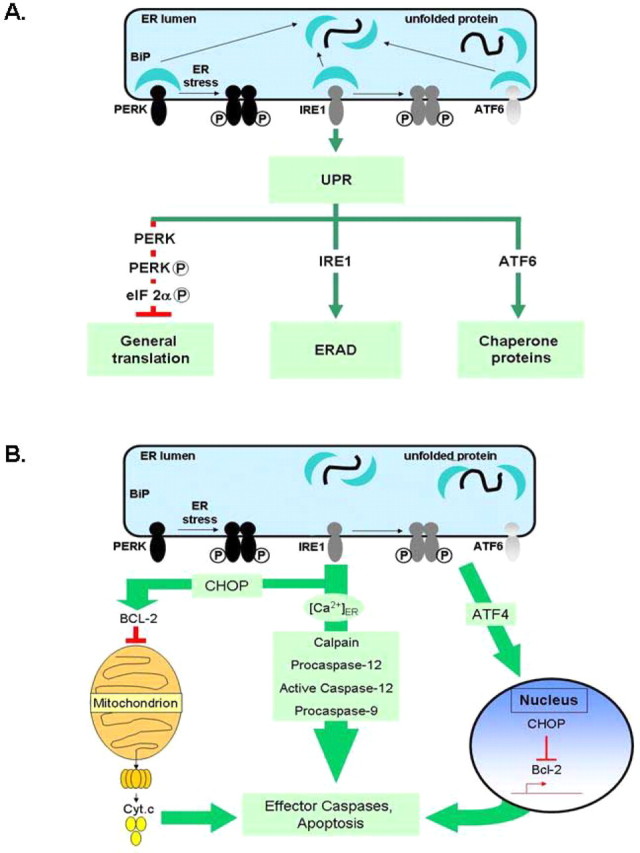
A, Simplified schematic showing the three major components of the unfolded protein response. Misfolded or unfolded proteins titrate BiP away from the three sensors of ER stress: PERK, IRE1, and ATF6. Activated PERK phosphorylates eIF2α to attenuate protein translation. Activated ATF6 leads to inductions of molecular chaperones BiP, protein disulfide isomerase (PDI), GRP94, etc. IRE1 activation leads to stimulation of protein degradation. The various ER chaperones, such as BiP and GRP94, are protective and control protein folding and components of the UPR. B, Sustained ER stress leads to proapoptotic signaling. Bcl-2 in conjunction with IP3 receptors (IP3-R) control ER calcium stores and release into the cytosol. Bcl-2 normally exerts an antiapoptotic function in the ER. CHOP inhibits Bcl-2, leading to calcium release; higher calcium levels sensitize mitochondria to other insults inducing cell death. ER specific caspases, such as the caspase-12, are thought to directly induce cell death.
