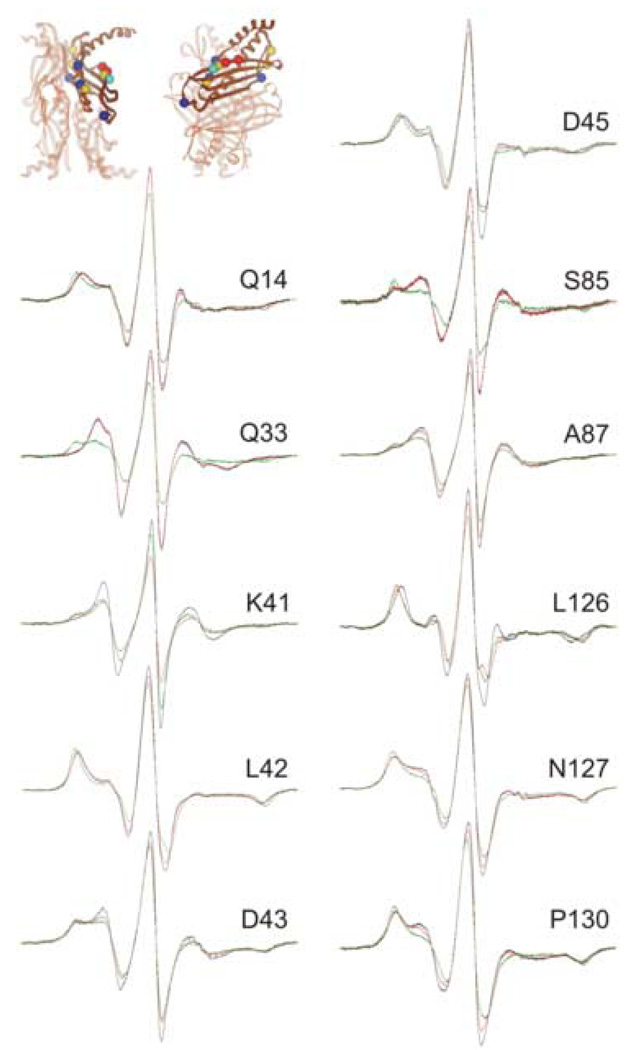
Figure 4.
Positions on SecB that show a high degree of constraint with precursor galactose-binding protein bound. The black traces are spectra of spin-labeled SecB alone, the red are spectra of an equimolar mixture of SecA and spin-labeled SecB and the green are an equimolar mixture of spin-labeled SecB and unfolded precursor galactose-binding protein. The positions of the residues examined are shown on the structure of SecB as spheres at the site of the α-carbon atom. The color indicates the nature of the original residue: gold, hydrophobic; blue, polar; bright blue, negative charge; red, positive charge. The structure in this and all subsequent structures is that generated by threading the E. coli sequence through the H. influenzae structure because the C-terminal residues are resolved. The PDB code for SecB is 1FX3.