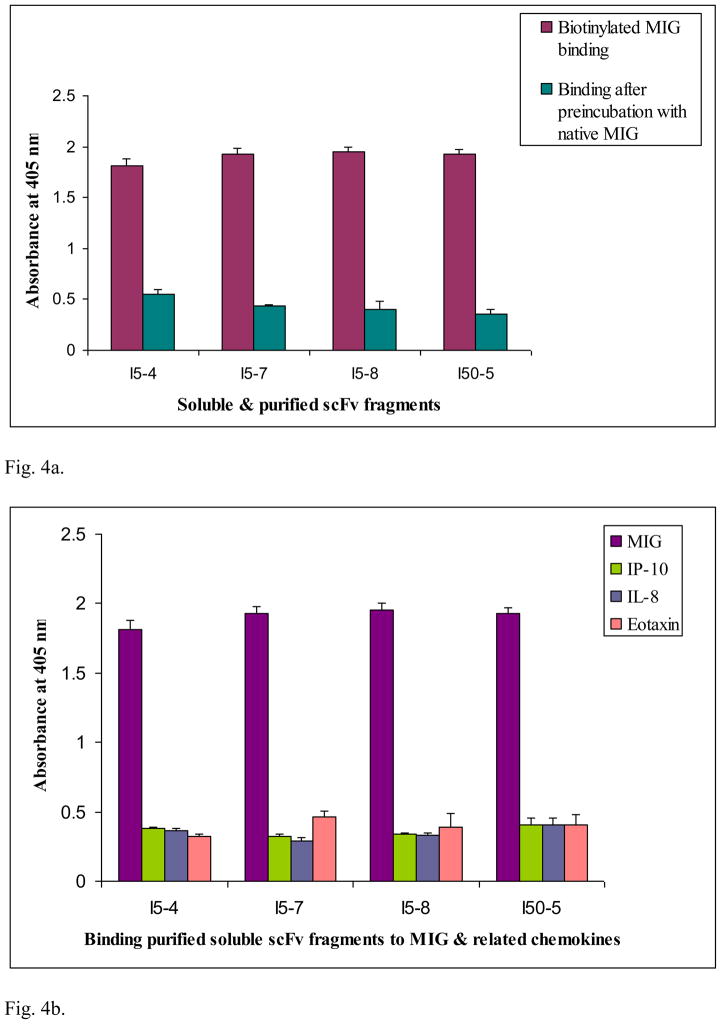
Fig. 4.
Fig. 4a. Competition ELISA determined binding properties of MIG recognizing soluble and purified scFv fragments (5 μg/mL) synthesized from selected clones isolated from Tomlinson I library. Each well was coated with a given soluble, purified scFv fragment (5 μg/mL or 1.7 × 10−7 M) in carbonate buffer; blocked with 3% (w/v) BSA in PBS, followed by addition of cold recombinant MIG (3.5 × 10−6 M) pre-incubated with the same soluble purified scFv; then biotinylated MIG (6.6 × 10−7 M) was added. The data represent mean ± standard deviation from duplicate measurements.
Fig. 4b. Differential binding to MIG and structurally related chemokines IP-10, NAP and Eotaxin by soluble and purified scFv fragments synthesized from selected clones (I5-4, I5-7, I5-8 and I50-5) isolated from Tomlinson I library. Each well was coated with a given soluble, purified scFv fragment (5 μg/mL or 1.7 × 10−7 M) in carbonate buffer; blocked with 3% (w/v) BSA in PBS, followed by addition of cold recombinant IP-10 or IL-8 (1.2 × 10−6 M) and cold recombinant Eotaxin (1.0 × 10−6 M), pre-incubated with the same soluble purified scFv, and then biotinylated MIG (6.6 × 10−7 M) was added; see text for further explanation of the results. The data represent mean ± standard deviation from duplicate measurements.