Figure 6.
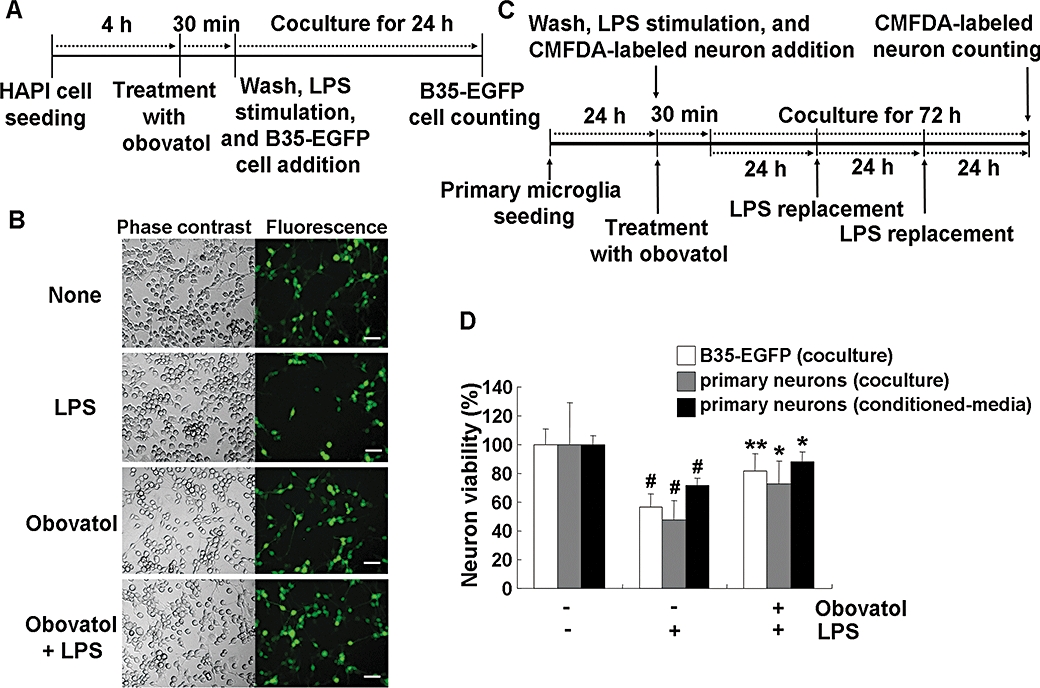
Obovatol suppressed microglial neurotoxicity. HAPI rat microglia cells were pretreated with 10 µM of obovatol for 30 min and washed with PBS. Then, LPS (100 ng·mL−1) and B35-EGFP rat neuroblastoma cells were added to microglia cells for the co-culture for 24 h (A). At the end of co-culture, the number of viable B35-EGFP neuroblastoma cells in the five randomly chosen microscopic fields per well was counted under a fluorescence microscope (D; white bars). Representative fluorescence or phase contrast images of cells are shown (B). Scale bar, 50 µm. Obovatol alone did not exert cytotoxicity against B35-EGFP neuroblastoma cells (data not shown). For the co-culture of primary microglia and primary neurons, microglia were pretreated with 10 µM of obovatol for 30 min. Then, LPS (100 ng·mL−1) and CMFDA-labelled primary neurons were added to microglia cultures. The culture media were replaced with fresh media containing LPS every 24 h, over 72 h (C). After LPS stimulation for 72 h, CMFDA-positive neurons were counted under a fluorescence microscope (D). For the preparation of primary microglia-conditioned media, microglia cultures were treated with LPS (100 ng·mL−1) in the absence or presence of 10 µM obovatol for 6 h. Culture media were removed, and fresh culture media were then added to microglia culture. After additional 24 h incubation, conditioned media were collected and added to primary neurons. After 24 h incubation, the viability of neurons was assessed by MTT assay (D). Results were expressed as a percentage of control (unstimulated microglia cultures+neuroblastoma or neurons) (mean ± SD; n= 3). #P < 0.01 versus control; *P < 0.05, **P < 0.01 versus LPS only. HAPI, highly aggressively proliferating immortalized; PBS, phosphate-buffered saline; LPS, lipopolysaccharide; EGFP, enhanced green fluorescent protein; CMFDA, 5-chloromethylfluorescein diacetate.
